4476
Evaluating High-Resolution High-b-value OGSE Time-dependent Diffusion in Human Brain at Ultra-high-gradient 3.0T1GE Research, Niskayuna, NY, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Walter Reed National Military Medical Center, Bethesda, MD, United States, 4Brigham and Women's Hospital, Boston, MA, United States, 5GE Healthcare, Stockholm, Sweden, 6Stanford University, Palo Alto, CA, United States
Synopsis
Keywords: Microstructure, Diffusion/other diffusion imaging techniques
Oscillating gradient spin echo (OGSE) time-dependent diffusion MRI has been shown to improve assessment of intra-cranial disease progression including glioma and multiple sclerosis. In human brain studies, however, high-resolution and high-b-value OGSE diffusion measurements suffer from low signal-to-noise ratio and Rician noise bias. In our preliminary studies with ultra-high-performance gradient 3.0T system, apparent diffusivity coefficient (ADC) of white matter at OGSE 35 Hz showed lower values when ADC was estimated from high b-values ≥1000 s/mm2 with 1.5-mm isotropic resolution, due to Rician noise bias. The effect of Rician noise bias on OGSE ADC measurement was reduced by using real-valued signals.INTRODUCTION
Characterizing tissue cytoarchitecture at the mesoscopic and microscopic scale is key to the assessment of intra-cranial disease progression including glioma1 and multiple sclerosis2. Emerging oscillating gradient spin echo (OGSE) time-dependent diffusion MRI provides image contrast sensitive to short tissue length scales, e.g., as in tumor cell size3 and assessment of axonal beading4. It has been demonstrated to improve the evaluation of tumor aggressiveness1 and treatment response5-7 in pre-clinical studies. In human brain, OGSE diffusion MRI is challenging. First, high OGSE frequency (i.e., short diffusion time) with adequate and/or high b-values8 are needed to provide high sensitivity to the restricted diffusion that reflects short tissue length scales. However, it can currently only be achieved at relatively long echo time (TE) in clinical whole-body MRI systems9-11. Further, high resolution is needed to improve lesion characterization in heterogenous tissue regions12 and to increase conspicuity of small lesions13. Simultaneously high-resolution, high b-value, and long echo time inevitably result in low signal-to-noise ratio and Rician noise bias, especially in OGSE time-dependent diffusion measurements. We proposed to evaluate OGSE time-dependent diffusion MRI measurements of human brain across a range of spatial resolutions and b-values with an ultra-high-performance gradient 3.0T MRI system.METHODS
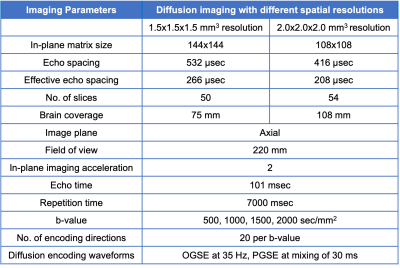
Data Acquisition: A healthy volunteer (female, 31 years old) was recruited and scanned under an IRB-approved protocol. MRI scan was performed in a 3.0T MRI system (MR750, GE Healthcare, USA) modified with a head-only MAGNUS14 gradient coil achieving 200 mT/m amplitude and 500 T/m/s slew rate and a 32-channel phased-array radiofrequency receive coil (Nova Medical, USA). Diffusion MRI at short and long diffusion times, implemented with cosine-modulated trapezoid OGSE15, 16 diffusion encoding at 35 Hz, and pulsed gradient spin echo (PGSE) diffusion encoding at a mixing time (Δ) of 30 ms, were acquired (Table 1). At each diffusion time, images with spatial resolutions of 1.5-mm and 2.0-mm isotropic at four b-values between 500 and 2000 s/mm2 were obtained. T1-weighted MPRAGE anatomical structural images were also acquired.Image reconstruction and analysis: To circumvent Rician noise bias, we estimated real-valued signals that maintain a Gaussian noise distribution after phase correction17. All diffusion images were corrected for geometric distortion and registered to T1-weighted anatomical images. Apparent diffusivity coefficient (ADC) at each diffusion time was calculated, after correction for diffusion gradient nonlinearity18. White matter and gray matter regions of all image slices were segmented based on T1-anatomical images19.
RESULTS
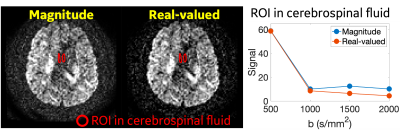
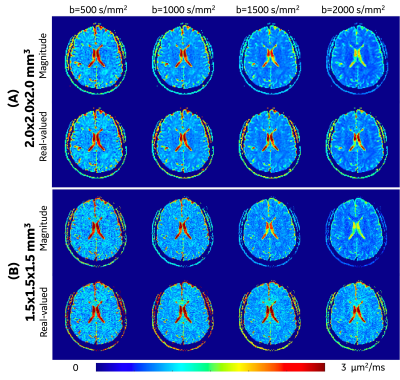
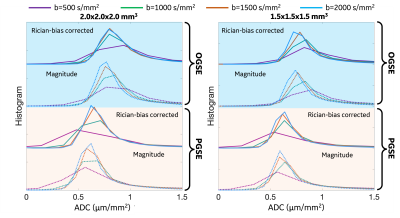
Rician noise bias correction: As the representative images (2.0x2.0x2.0 mm3 at OGSE 35 Hz) shown in Figure 1, the real-valued image showed reduced noise compared to the magnitude image. In the regions-of-interest of cerebrospinal fluid in this image slice (red contour), the real-valued spherical-averaged signals decayed to ~0 at high b-values, while the magnitude signals were elevated and maintained a non-zero floor due to Rician noise bias.Figure 2 shows representative ADC maps at OGSE 35 Hz estimated from different b-values and different resolutions. ADC measurements of white matter and gray matter showed similar image contrast between estimations using magnitude and real-valued signals for all the four b-values at 2.0x2.0x2.0 mm3 (Figure 2A), and for b-value=500 s/mm2 at 1.5x1.5x1.5 mm3 (Figure 2B, 1st column). ADC measurements of white matter showed significantly different image contrast between estimates using magnitude signals and real-valued signals for high b-values ≥1000 s/mm2 at high resolution of 1.5x1.5x1.5 mm3 (Figure 2B, 2-4 columns).
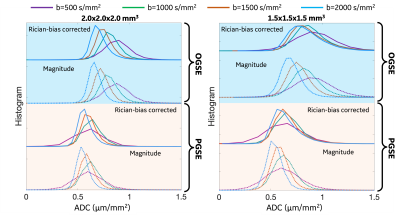
Whole-brain white matter (Figure 3): At both resolutions, ADC measurements obtained from signals at high b-values were lower and the change of ADC measurements was more obvious at 2.0x2.0x2.0 mm3. In real-valued signal-estimated ADC measurements (solid lines), ADC at OGSE 35 Hz was higher, compared to ADC obtained from signals at the same b-value at PGSE 30 ms.
Whole-brain gray matter (Figure 4): At both resolutions, ADC measurements at OGSE 35 Hz obtained from signals at all the four b-values showed similar values. In real-valued signal-estimated ADC measurements (solid lines), ADC at OGSE 35 Hz was higher, compared to ADC obtained from signals at the same b-value at PGSE 30 ms.
DISCUSSION AND CONCLUSION
We preliminarily evaluated time-dependent diffusivities of human brain across a range of spatial resolutions and high b-values with an ultra-high-performance gradient 3.0T MRI system. At a relatively long TE>100 ms, moderate resolution (e.g., 2.0x2.0x2.0 mm3), and a relatively high b-value of 2000 s/mm2, ADC measurements obtained from magnitude signals and real-valued signals showed consistent results in white matter. At high resolution (e.g., 1.5x1.5x1.5 mm3) and relatively high b-values ≥1000 s/mm2, ADC measurements obtained from magnitude signals were affected by Rician noise bias, which was reduced by using the real-valued signals.Similar to the previous study20, we observed decreased ADC measurements of white matter obtained from signals at high b-values, compared to that at low b-values. We note that Rician noise bias needs to be corrected for, at a high resolution of 1.5x1.5x1.5 mm3 where signal-to-noise ratio is low.
In conclusion, we demonstrated the need to correct for Rician noise bias in high-resolution and high-b-value OGSE diffusion MRI. Preliminary clinical studies are planned to evaluate the performance of optimized high-resolution and high-b-value OGSE diffusion MRI in brain tumor and in multiple sclerosis patients.
Acknowledgements
The authors acknowledge the funding support from CDMRP W81XWH-16-2-0054, and Massachusetts Life Science Foundation. The opinions or assertions contained herein are the views of the authors and are not to be construed as the views of the U.S. Department of Defense, Walter Reed National Military Medical Center, or the Uniformed Services University.References
1. Reynaud O. Time-Dependent Diffusion MRI in Cancer: Tissue Modeling and Applications. Frontiers in Physics. 2017;5(58). doi: 10.3389/fphy.2017.00058.
2. Lee H-H, Papaioannou A, Kim S-L, Novikov DS, Fieremans E. A time-dependent diffusion MRI signature of axon caliber variations and beading. Communications biology. 2020;3(1):1-13.
3. Xu J. Probing neural tissues at small scales: Recent progress of oscillating gradient spin echo (OGSE) neuroimaging in humans. Journal of Neuroscience Methods. 2021;349:109024. doi: https://doi.org/10.1016/j.jneumeth.2020.109024.
4. Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proceedings of the National Academy of Sciences. 2014;111(14):5088-93.
5. Jiang X, Xu J, Gore JC. Quantitative temporal diffusion spectroscopy as an early imaging biomarker of radiation therapeutic response in gliomas: A preclinical proof of concept. Advances in Radiation Oncology. 2019;4(2):367-76. doi: https://doi.org/10.1016/j.adro.2018.11.003.
6. Portnoy S, Fichtner ND, Dziegielewski C, Stanisz MP, Stanisz GJ. In vitro detection of apoptosis using oscillating and pulsed gradient diffusion magnetic resonance imaging. NMR in Biomedicine. 2014;27(4):371-80. doi: https://doi.org/10.1002/nbm.3070.
7. Roberts TA, Hyare H, Agliardi G, Hipwell B, d’Esposito A, Ianus A, Breen-Norris JO, Ramasawmy R, Taylor V, Atkinson D. Noninvasive diffusion magnetic resonance imaging of brain tumour cell size for the early detection of therapeutic response. Scientific reports. 2020;10(1):1-13.
8. Portnoy S, Flint JJ, Blackband SJ, Stanisz GJ. Oscillating and pulsed gradient diffusion magnetic resonance microscopy over an extended b-value range: Implications for the characterization of tissue microstructure. Magnetic Resonance in Medicine. 2013;69(4):1131-45. doi: https://doi.org/10.1002/mrm.24325.
9. Wu D, Jiang K, Li H, Zhang Z, Ba R, Zhang Y, Hsu Y-C, Sun Y, Zhang Y-D. Time-Dependent Diffusion MRI for Quantitative Microstructural Mapping of Prostate Cancer. Radiology.0(0):211180. doi: 10.1148/radiol.211180. PubMed PMID: 35258368.
10. Xu J, Jiang X, Devan SP, Arlinghaus LR, McKinley ET, Xie J, Zu Z, Wang Q, Chakravarthy AB, Wang Y. MRI‐cytometry: Mapping nonparametric cell size distributions using diffusion MRI. Magnetic resonance in medicine. 2021;85(2):748-61.
11. Iima M, Kataoka M, Honda M, Ohashi A, Ohno Kishimoto A, Ota R, Uozumi R, Urushibata Y, Feiweier T, Toi M, Nakamoto Y. The Rate of Apparent Diffusion Coefficient Change With Diffusion Time on Breast Diffusion-Weighted Imaging Depends on Breast Tumor Types and Molecular Prognostic Biomarker Expression. Investigative Radiology. 2021;56(8):501-8. doi: 10.1097/rli.0000000000000766. PubMed PMID: 00004424-202108000-00005.
12. Hu LS, Hawkins-Daarud A, Wang L, Li J, Swanson KR. Imaging of intratumoral heterogeneity in high-grade glioma. Cancer Letters. 2020;477:97-106. doi: https://doi.org/10.1016/j.canlet.2020.02.025.
13. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, Wallin M, Helme A, Angood Napier C, Rijke N, Baneke P. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Multiple Sclerosis Journal. 2020;26(14):1816-21. doi: 10.1177/1352458520970841. PubMed PMID: 33174475.
14. Foo TK, Tan ET, Vermilyea ME, Hua Y, Fiveland EW, Piel JE, Park K, Ricci J, Thompson PS, Graziani D. Highly efficient head‐only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0 T (MAGNUS) for brain microstructure imaging. Magnetic resonance in medicine. 2020;83(6):2356-69.
15. Tan ET, Shih RY, Mitra J, Sprenger T, Hua Y, Bhushan C, Bernstein MA, McNab JA, DeMarco JK, Ho VB. Oscillating diffusion‐encoding with a high gradient‐amplitude and high slew‐rate head‐only gradient for human brain imaging. Magnetic resonance in medicine. 2020;84(2):950-65.
16. Shih R, Zhu A, DeMarco JK, Morris HD, Hood M, Abad N, Madhavan R, Marinelli L, Foo T, Ho VB, editors. Initial Clinical Experience with MAGNUS Ultra-High-Performance Gradient Coil for Diffusion Microstructure Imaging of Intracranial Pathology. Proc Intl Soc Mag Reson Med 30; 2022; London, United Kingdom.
17. Sprenger T, Sperl JI, Fernandez B, Haase A, Menzel MI. Real valued diffusion-weighted imaging using decorrelated phase filtering. Magnetic Resonance in Medicine. 2017;77(2):559-70. doi: https://doi.org/10.1002/mrm.26138.
18. Tan ET, Marinelli L, Slavens ZW, King KF, Hardy CJ. Improved correction for gradient nonlinearity effects in diffusion‐weighted imaging. Journal of Magnetic Resonance Imaging. 2013;38(2):448-53.
19. Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An Open Source Multivariate Framework for n-Tissue Segmentation with Evaluation on Public Data. Neuroinformatics. 2011;9(4):381-400. doi: 10.1007/s12021-011-9109-y.
20. Michael ES, Hennel F, Pruessmann KP. Evaluating diffusion dispersion across an extended range of b-values and frequencies: Exploiting gap-filled OGSE shapes, strong gradients, and spiral readouts. Magnetic Resonance in Medicine.n/a(n/a). doi: https://doi.org/10.1002/mrm.29161.
Figures