4470
Radiomics-Based Classification of Severe Pulmonary Regurgitation in Patients with Repaired Tetralogy of Fallot by Cardiac Magnetic Resonance1Department of Biomedical Engineering and Environmental Sciences, National Tsing Hua University, Hsinchu, Taiwan, 2Department of Radiology, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, 3Department of Electrical Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan, 4Department of Automatic Control Engineering, Feng Chia University, Taichung, Taiwan, 5Department of Pediatrics, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, 6Dpartment of Pediatrics, National Yang Ming Chiao Tung University, Taipei, Taiwan
Synopsis
Keywords: Heart, Radiomics
Cardiac magnetic resonance (CMR) radiomics is a novel technique for advanced cardiac image phenotyping. This study aimed to develop a radiomics-based classification model by CMR images and thereby to identify rTOF patients with severe pulmonary regurgitation (PR). In our results, the radiomics-based classification model can successfully identify rTOF patients with PR ≥25% by routine CMR cine images. The extracted features underlined the RV intracardiac flow alteration due to PR and the potential right ventricular remodeling in rTOF patients with severe PR. This radiomics-based information may be helpful in determining the appropriate timing for pulmonary valve replacement in the future.Introduction
Chronic pulmonary regurgitation (PR) can lead to right ventricular (RV) volume overload and dysfunction in patients with repaired tetralogy of Fallot (rTOF), resulting in an increase in morbidity and mortality1-3. Radiomics is an emerging image analysis technique which has been used in cardiac magnetic resonance (CMR) images for a deeper phenotyping of cardiovascular health and diseases4. Compared to conventional CMR indices, radiomics analysis can extract representative imaging features to reveal cardiac abnormality by examining plenty of complex and subtle characteristics regarding cardiac morphology and tissue properties at various scales and locations5-9.This study aimed to develop a radiomics-based classification model by CMR images to identify rTOF patients with severe PR.
Methods
The study population comprised 40 patients with rTOF (age=22.5±3.6 years, 18 females). A cut-off value of severe PR was set as ≥25% according to the ACC/AHA 2008 guidelines 10. rTOF patients were divided into two subgroups. rTOF1 group (n=12) and rTOF2 group (n=28) were patients with PR < 25% and ≥ 25%, respectively.CMR images were acquired in a 3T scanner (Skyra, Siemens). The scanning parameters of cine balanced steady-state free procession were TR/TE=3.2/1.7 ms, flip angle=50°, voxel size=1.25x1.25x8 mm3, temporal resolution=46.8 ms (interpolated to 25 phases/cardiac cycle), a short-axis stack with 10-12 slices covering left and right ventricles (LV, RV) from base to apex with breath-hold and retrospective ECG-gating techniques. The LV and RV endocardial contours in LV and RV and the epicardial contours in LV were delineated automatically at end-diastolic (ED) and end-systolic (ES) phases using an institute-developed tool. The regions of interest (ROIs) for radiomics analysis were RV blood pool, LV blood pool, and LV myocardium (LVMYO).
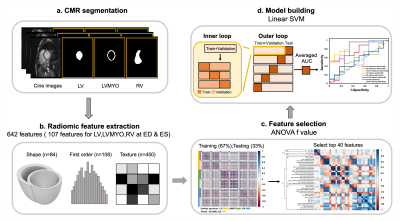
Figure 1 illustrates the radiomics workflow. Pyradiomics (version 1.3.0)11 was used to automatically extract radiomic features. 642 radiomic features were extracted, consisting of 107 radiomic features in LV, RV and LVMYO at two cardiac phases of ED and ES. The features in shape and first order categories quantified morphological characteristics and histogram-based signal intensity characteristics, respectively. The features in texture analysis enable the detection of spatial inter-pixel interactions by using advanced matrix analysis.
The ANOVA f-value was used to select the top 40 features for the establishment of classification model12. A linear support vector machine (SVM) model was used for classification13,14. We performed a five-fold outer loop and a three-fold inner loop in the SVM model. In the outer loop, 80% and 20% of the data were set as training+validation and testing datasets, respectively. In the inner loop, the datasets were divided into 67% and 33% for training and validation, respectively. CMR indices or radiomics-based features or a combination of CMR indices and radiomics-based features were employed to establish 7 classification models for differentiation rTOF2 from rTOF1 patients. Student t test and Pearson correlation were performed when appropriate. A p value <0.05 was considered statistically significant.
Results
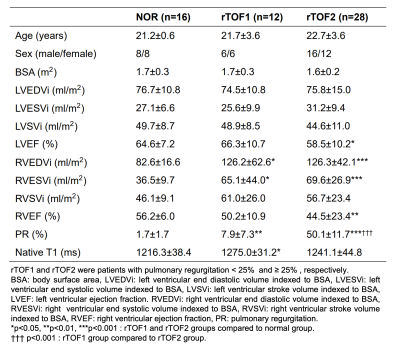
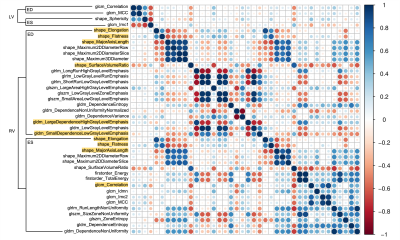
The demographic and cardiovascular characteristics in rTOF1 and rTOF2 groups were summarized in Table 1. Except for PR (p<0.001), CMR indices had no significant difference between rTOF1 and rTOF2 groups.In the correlogram (Figure 2), 36 of top 40 (90%) selected features belonged to the RV features and were weakly correlated (r<0.9) with each other, not containing redundant information.
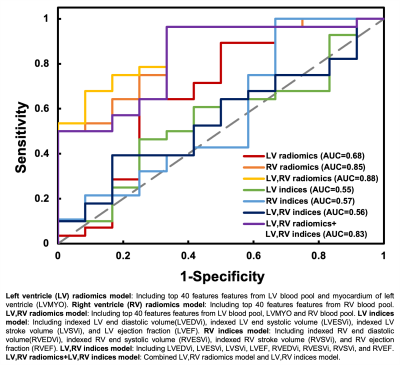
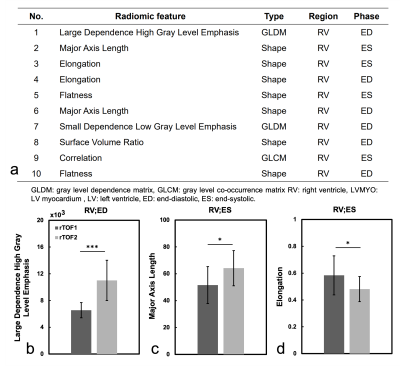
Figure 3 illustrates the receiver operation characteristic (ROC) curves to differentiate rTOF2 patients from rTOF1 patients with 7 different models. The area under curve (AUC) of averaged 5 folds in 7 models were ranged from 0.55 to 0.88. Of note, LV radiomic features included features from LV blood pool and LVMYO. Figure 4a lists the 10 best-performing radiomic features, sorted by ANOVA f-value, for identification of the severity of PR in rTOF patients.
Figures 4(b-d) show the values of the top three radiomic features in rTOF1 and rTOF2 groups. Compared to rTOF1 group, the rTOF2 group presented higher large dependence high gray level emphasis (LDHGLE) in RV at ED (p<0.001) and major axis length in RV at ES (p=0.011). The rTOF2 group exhibited lower elongation than rTOF1 group in RV at ES (p=0.013).
Discussion and Conclusion
The proposed radiomics-based classification models established by CMR cine images demonstrated high AUC to differentiate rTOF2 patients from rTOF1 patients. Compared to conventional CMR indices, radiomics-based models enable the improvements of identification of the severity of PR in rTOF patients. The performance of radiomics-based models was not improved while combined with conventional CMR indices.The high LDHGLE feature in RV at ED in rTOF2 group might describe the altered intra-RV flow with impaired diastolic inflow through the tricuspid valve15 and reduced diastolic vorticity16 due to severe PR. The major axis length and elongation, belonging to shape category, in RV at ES in rTOF2 group significantly differed from that in rTOF1 group, suggesting more sever RV remodeling in rTOF2 group. Of note, this radiomics-based alteration was revealed before significant differences in cardiac volumetric measurements between these two subgroups.
In conclusion, the radiomics-based classification model can successfully identify rTOF patients with severe PR by routine CMR cine images. The extracted features underlined the RV intracardiac flow alteration due to PR and the more severe RV remodeling in rTOF2 patients with severe PR.
Acknowledgements
No acknowledgement found.References
1. Wessel HU, et al. Exercise performance in tetralogy of Fallot after intracardiac repair. J Thorac Cardiovasc Surg. 1980.
2. Carvalho JS, et al. Exercise capacity after complete repair of tetralogy of Fallot: deleterious effects of residual pulmonary regurgitation. Br Heart J. 1992.
3. Gatzoulis MA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000.
4. Raisi-Estabragh Z, et al. Cardiac magnetic resonance radiomics: basic principles and clinical perspectives. Eur Heart J Cardiovasc Imaging. 2020.
5. Cetin I, et al. Radiomics signatures of cardiovascular risk factors in cardiac MRI: results from the UK biobank. Front Cardiovasc Med. 2020.
6. Amano Y, et al. Relationship between extension or texture features of late gadolinium enhancement and ventricular tachyarrhythmias in hypertrophic cardiomyopathy. Biomed Res Int. 2018.
7. Aerts HJWL, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014.
8. Neisius U, et al. Radiomic analysis of myocardial native T1 imaging discriminates between hypertensive heart disease and hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2019.
9. Cheng S, et al. LGE-CMR-derived texture features reflect poor prognosis in hypertrophic cardiomyopathy patients with systolic dysfunction: preliminary results. Eur Radiol. 2018.
10. Warnes CA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008.
11. van Griethuysen JJM, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017.
12. Izquierdo C, et al. Radiomics-Based Classification of Left Ventricular Non-compaction, Hypertrophic Cardiomyopathy, and Dilated Cardiomyopathy in Cardiovascular Magnetic Resonance. Front Cardiovasc Med. 2021.
13. Martin-Isla C, et al. Image-based cardiac diagnosis with machine learning: a review. Front Cardiovasc Med. 2020.
14. Rauseo E, et al. New imaging signatures of cardiac alterations in ischemic heart disease and cerebrovascular disease using CMR radiomics. Front Cardiovasc Med. 2021.
15. Mikhail A, et al. How pulmonary valve regurgitation after tetralogy of fallot repair changes the flow dynamics in the right ventricle: An in vitro study. Med Eng Phys. 2020.
16. Loke YH, et al. Computational Modeling of Right Ventricular Motion and Intracardiac Flow in Repaired Tetralogy of Fallot. Cardiovasc Eng Technol. 2022
Figures