4459
No separate acquisition timing scheme is necessary for the MOLLI technique when used for the post contrast cardiac T1 mapping
Seonghwan Yee1
1Radiology, Massachusetts General Hospital, Boston, MA, United States
1Radiology, Massachusetts General Hospital, Boston, MA, United States
Synopsis
Keywords: Heart, Relaxometry, MOLLI
The modified Look-Locker imaging (MOLLI) technique, widely used in cardiac T1 mapping, requires specification of an acquisition timing scheme. For better quantification of wide range of T1 values, different acquisition schemes have been used for the prior- and post-contrast conditions. However, the strategy of having two different acquisition schemes might add complexity to the consistent management of clinical protocols, especially in a clinical environment with multiple MRI systems. Hence, a phantom experiment was performed to examine the necessity of this strategy. The results here suggest that having a separate acquisition scheme for the post-contrast condition would not be necessary.Introduction
When cardiac T1 mapping is performed with the MOLLI technique (1), different acquisition timing schemes are applied separately for the non-contrast native (or long T1) and the post-contrast enhanced (or short T1) conditions. It is mainly because of the two reasons. First, the significantly shortened post-contrast T1 values are thought to be better measured by a more rapid acquisition timing scheme in the early dynamic recovery phase of the inverted magnetization. Second, when compared to the tissue with relatively long T1 in the non-contrast native condition, the short T1 tissue would sufficiently recover within the shorter time before the next inversion pulse. For example, the native (long) acquisition scheme could be the 5s of initial acquisition after the first inversion, followed by the 3s of rest and 3s of the last acquisition after the second inversion, which could be noted as 5s(3s)3s. With the similar notation, the enhanced (short) scheme could be 4s(1s)3s(1s)2s. However, in a clinical environment with multiple MRI systems, the distinction and application of two separate acquisition timing schemes, namely the long and the short schemes, may unnecessarily add complexity to the prompt—and consistent—optimization of the cardiac T1 mapping protocols and propagation of them across multiple systems. Hence, a phantom experiment was performed to compare the two different acquisition schemes for a wide range of T1 values.Methods
The MRI phantom: A T1 phantom was built using 12 vials (each of 50 mL capacity and 4.8 cm2 cross-sectional area) containing distilled water mixed with different concentrations of gadolinium contrast agent (gadobenate dimeglumine). The prepared concentration ranged from 0.03 mg/mL to 0.7 mg/mL to make the T1 of each vial in the range approximately from 200 to 2000 ms.MRI scan: MRI scans were performed in a 1.5 T MRI system (Ingenia, Philips, Netherlands). The T1 phantom was first scanned with the IR-based sequence (TI= 50 to 2800 ms, TR=6 s, TE= 8 ms) to determine the T1 value of each vial, which was then taken as a reference T1 at the time of the experiment. Then, the MOLLI technique was applied with the scanner-provided simulation of the ECG trigger. The simulated ECG signal was generated at a fixed heart rate (HR) of 100 bpm and also at different HRs ranging from 40 to 160 bpm in the steps of 20 bpm. For each HR, both long and short acquisition schemes were applied, and they were 5s(3s)3s and 4s(1s)3s(1s)2s, respectively. All the analyses for determining T1 values were performed using the MATLAB-based in-house program, by which the signal change from each vial was sampled and fitted to the 3-parameter exponential T1 modeling equation (2).
Results
The T1 values of the phantom, ranging from 216 to 2017 ms in the 12 vials, are shown as a map in Fig. 1. The signal changes detected for all the vials by the MOLLI technique with the simulated HR of 100 bpm are shown in Fig. 2, where the curves for the long and the short acquisition schemes are separated into (a) and (b). Shown in Fig. 3 are the MOLLI-determined T1 values compared to the reference T1 values, for the HR of 100 bpm, with the long and the short acquisition schemes. As expected, the short acquisition scheme failed to accurately detect the long T1 values. In this case, the deviation started from the T1 value of approximately 1000 ms, and starting from the T1 value of approximately 1500 ms, the error becomes 10% or more. However, the long acquisition scheme did not show any clear deviation from the reference values even for the short T1 values less than 400 ms, and the errors over the whole tested T1 range was consistently less than 2%. The comparisons made with a separate set of measurements at different HRs are also shown in Fig. 4. For all different HRs, the short (enhanced) acquisition scheme, as expected, deviated from the reference in the higher T1 values, and even in the short T1 value range (< 400 ms), it did not show a clear advantage compared to the long (native) acquisition scheme.Discussion
The short (or enhanced) scheme is designed to provide the better accuracy in measuring the shortened T1 values in the heart after contrast injection. However, the results here suggest that the accuracy of the short scheme is in fact not better than the long (native) scheme. In addition, the strategy of having two different acquisition schemes, to the author’s experience as a clinical MRI physicist, have added complexity to the consistent management of multiple clinical protocols and across multiple MRI systems. In this regard, the possible single acquisition scheme strategy may help reduce the complexity. Another suggestion from the results here is that the acquisition scheme does not need to be tailored by the post-contrast time and the single fixed acquisition scheme could be used even with a very late post-contrast time.Conclusion
In the cardiac T1 mapping utilizing the MOLLI technique, the single acquisition timing scheme, namely the long (native) scheme, can be used for both prior- or post-contrast conditions without sacrificing the measurement accuracy.Acknowledgements
No acknowledgement found.References
1. Messroghli DR, et al., Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart, Magn Reson Med, 52:141-146 (2004)
2. Taylor AJ, et al., T1 mapping: basic techniques and clinical applications, JACC: Cardiovascular Imaging, 9(1):67-81 (2016)
Figures
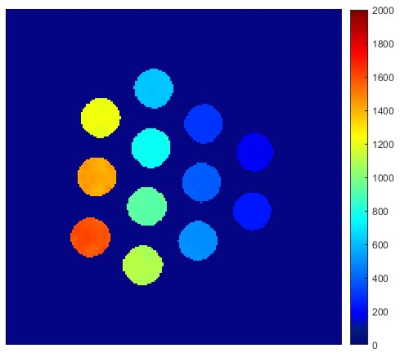
Figure 1: T1 map of the phantom used in this study. The T1 values of all the vials ranged from 216 ms to 2017 ms. See the text for the phantom description.
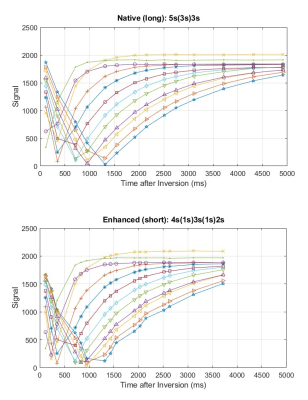
Figure 2: The signal curves for all the vials of the T1 phantom, acquired by the MOLL technique with the simulated ECG signal at the heart rate of 100 bpm. The top curves, (a), were acquired with the native (long) acquisition scheme and the bottom curves, (b), were acquired with the enhanced (short) acquisition scheme.
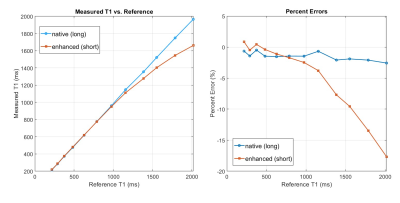
Figure 3: The comparison of the T1 values of all the vials of the phantom measured by the MOLLI technique to the reference values measured by the IR-based technique, for the simulated heart rate of 100 bpm. The percent error is shown in the right. The native (long) acquisition scheme shows a consistent low % error over the range covering all the T1 values of the phantom.
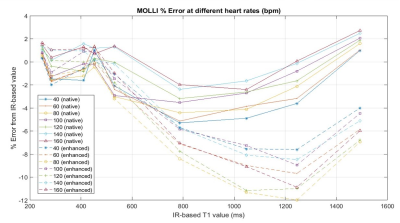
Figure 4: The % errors when the T1 values of the phantom acquired by the MOLLI technique with several different simulated heart rates were compared to the reference values. The native (long) acquisition scheme shows a consistent comparable accuracy even in the low T1 range (less than 500 ms) at various simulated heart rates.
DOI: https://doi.org/10.58530/2023/4459