4456
Intra-subject repeatability and initial clinical evaluation of approximate myocardial spin-lock dispersion mapping
Joao Tourais1, Yidong Zhao1, Iain Pierce2, Christian Nitsche2, George D Thornton2, Mehmet Akcakaya3, Qian Tao1, Thomas A Treibel2, and Sebastian Weingartner1
1Imaging Physics, Delft University of Technology, Delft, Netherlands, 2Barts Heart Centre and Institute for Cardiovascular Science, University College London, London, United Kingdom, 3Department of Electrical and Computer Engineering and Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
1Imaging Physics, Delft University of Technology, Delft, Netherlands, 2Barts Heart Centre and Institute for Cardiovascular Science, University College London, London, United Kingdom, 3Department of Electrical and Computer Engineering and Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Keywords: Myocardium, Quantitative Imaging
Approximate myocardial spin-lock (SL) dispersion mapping was obtained using simultaneous TRAFF2 and T2 mapping with good image quality and consistent repeatability among healthy subjects at 3T. Initial patient data indicates clinical feasibility and sensitivity to scar tissue, as identified by LGE. T2 and TRAFF2 are sensitive to distinct macromolecular interactions, showing differential contrast in disease. SL dispersion may exploit this and allow for isolating contrast components (e.g. chemical exchange effects). Our results suggest that these may allow for robust, complementary, and comprehensive clinical sensitivity to pathological remodeling. Future evaluation in a larger patient cohort with different myocardial injuries is warranted.INTRODUCTION
Spin-Lock (SL) dispersion has shown promising results as a non-contrast imaging biomarker for the detection of myocardial infarction (MI) due to increased sensitivity compared to conventional relaxometry1. However, SL pulses (e.g. T1ρ) are often limited by the deposited specific absorption rate (SAR) and are sensitive to field inhomogeneities2. This hampers its use for cardiac applications, especially at high field strengths (3T and above). An alternative to T1ρ is Relaxation Along a Fictitious Field in the 2nd rotating frame (RAFF2), which achieves spin-locking with a lower SAR3,4. Recently, a method for simultaneous quantification of T2 and TRAFF2 has been proposed for approximate myocardial SL dispersion mapping in a single breath-hold (BH)5. Here, we evaluate this method in phantom and healthy subjects at 3T. Intra-subject repeatability and early clinical feasibility were assessed in patients with different myocardial injuries.METHODS
Approximate SL dispersion was acquired in phantom (T1MES phantom6), 8 healthy subjects (6 males; 35±4y), and 3 patients (3 males; 63±8y) with suspected cardiac diseases on a 3T scanner (Magnetom Prisma, Siemens). Simultaneous TRAFF2 and T2 mapping (Figure 1) was performed by acquiring five images in a single 16s BH, as previously proposed5: 1 image with no preparation, 2 RAFF2-prepared images of different duration (TRAFF2p=12.9 and 25.7ms), 1 T2-prep image (T2p=50ms). Each of these images is preceded by a 4s rest period. Finally, a saturation-prepared image is acquired to capture the effect of imaging pulses on the magnetization curve7. TRAFF2 and T2 maps were generated using a 4-parameter fit5 (Figure 1) following groupwise image registration8. Approximate SL dispersion maps were calculated by subtracting T2 and TRAFF2, normalized by the RAFF frequency (peak RF pulse power=625Hz). In phantoms and in vivo, simultaneous TRAFF2 and T2 mapping used bSSFP with the following parameters: FOV: 270x340mm2; resolution: 1.8x1.8mm2; Slice thick.: 8mm; GRAPPA: 2; FA: 70°. Deep-learning-based myocardial segmentation9 was performed and mean relaxation times were extracted. To assess repeatability, approximate SL dispersion mapping was repeated three times in separate acquisitions, and the coefficient of variation (CoV) was calculated. In patients, the infarct area was visually assessed on the quantitative maps and compared to conventional native MOLLI T1 mapping and post-contrast late-gadolinium enhancement (LGE).RESULTS
TRAFF2, T2, and SL dispersion maps show good sensitivity to changes in phantom composition (Figure 2). The vials range was 102.0-552.3ms (TRAFF2), 36.6-218.6ms (T2), and 101.2-533.9ms2 (SL Dispersion). Excellent intra-scanner repeatability was obtained across the three repetitions for all nine vials with an average CoV of 1.4±0.8% (0.6-3.3%), 1.0±0.5% (0.6-2.0%), and 2.1±1.2% (0.9-4.7%) for TRAFF2, T2, and SL dispersion, respectively (Figure 2B). Figure 3 shows representative maps acquired in healthy subjects, exhibiting overall high visual map quality and a good depiction of the myocardium. In healthy subjects, simultaneous TRAFF2 (80.8±7.4ms, CoV 19.6±8.3%), T2 (40.8±0.8ms, CoV 12.1±7.8%), and approximate SL dispersion (63.9±11.1ms2, CoV 41.3±18.9%) were obtained (Figure 4). Quantitative maps of a patient with MI are shown in Figure 5. Scar tissue is visible in the inferior segment of LGE, TRAFF2, and approximate SL dispersion maps, while in native T1 and T2, no myocardial injury is visually apparent.DISCUSSION
In this study, we explored the repeatability and clinical feasibility of myocardial TRAFF2, T2, and approximate SL dispersion mapping at 3T. High visual map quality with consistent repeatability was achieved in healthy subjects and initial evidence of sensitivity to myocardial injury was obtained.Compared to constant-amplitude SL pulses, the higher tolerance of RAFF pulses to B0 and B1+ field inhomogeneities enabled homogenous mapping of TRAFF2 in all myocardial segments of the three acquired short-axis slices among healthy subjects. Due to the nested sine-amplitude and cosine-frequency modulated RF pulses, RAFF operates in a sub-adiabatic regime. This makes RAFF a viable alternative to conventional SL pulses, especially in cardiac imaging at high field strengths.
Initial approximate myocardial SL dispersion mapping patient data suggest clinical feasibility and sensitivity to scar tissue, as identified by LGE. The combination of approximate SL dispersion, with T2 and TRAFF2, could be used as a non-contrast alternative to LGE. This may allow for clinical use in patients with renal dysfunction or allergies to gadolinium-based contrast agents. For the detection of myocardial diseases, this may allow for complementary and comprehensive clinical sensitivity to pathological remodeling in a single scan. Assessment of RAFF2 and spin-spin relaxation times is particularly attractive due to the potential differentiation between acute and chronic myocardial injury, as well as the detection of diffuse myocardial fibrosis. In approximate SL dispersion, by subtracting T2 (0Hz) from TRAFF2 (625Hz), the intrinsic effect of spin-spin interaction is substantially reduced, eliminating contributions that affect both contrasts (e.g. increased relaxation times in the presence of edema or free fluid). Thus, SL dispersion may reflect isolated contrast components, such as chemical exchange effects (between free water protons and protons bound with macromolecules) more faithfully, providing a potential sensitivity and specificity to excessive collagen deposition.
CONCLUSION
Our results indicate that myocardial spin-lock dispersion mapping is a promising candidate for non-contrast detection of myocardial injury. High visual map quality was achieved with a single breath-hold method in phantom and in vivo, with consistent intra-subject repeatability. Initial evidence of clinical sensitivity to pathological remodeling warrants further exploration in a larger cohort of patients.Acknowledgements
S.W. acknowledges funding from the 4TU Precision Medicine program, an NWO Start-up STU.019.024, and ZonMW OffRoad 04510011910073.References
- Han Y, Liimatainen T, Gorman RC, Witschey WR. Assessing Myocardial Disease Using T1rho MRI. Curr Cardiovasc Imaging Rep 2014;7(2):9248.
- Witschey WR, 2nd, Borthakur A, Elliott MA, Mellon E, Niyogi S, Wallman DJ, Wang C, Reddy R. Artifacts in T1 rho-weighted imaging: compensation for B(1) and B(0) field imperfections. J Magn Reson 2007;186(1):75-85.
- Liimatainen T, Sorce DJ, O'Connell R, Garwood M, Michaeli S. MRI contrast from relaxation along a fictitious field (RAFF). Magn Reson Med 2010;64(4):983-994.
- Yla-Herttuala E, Laidinen S, Laakso H, Liimatainen T. Quantification of myocardial infarct area based on TRAFFn relaxation time maps - comparison with cardiovascular magnetic resonance late gadolinium enhancement, T1rho and T2 in vivo. J Cardiovasc Magn Reson 2018;20(1):34.
- Tourais J, Demirel OB, Tao Q, Pierce I, Thornton GD, Treibel TA, Akcakaya M, Weingartner S. Myocardial Approximate Spin-lock Dispersion Mapping using a Simultaneous T2 and TRAFF2 Mapping at 3T MRI. Annu Int Conf IEEE Eng Med Biol Soc 2022;2022:1694-1697.
- Captur G, Gatehouse P, Keenan KE, Heslinga FG, Bruehl R, Prothmann M, Graves MJ, Eames RJ, Torlasco C, Benedetti G, Donovan J, Ittermann B, Boubertakh R, Bathgate A, Royet C, Pang W, Nezafat R, Salerno M, Kellman P, Moon JC. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance-the T1 Mapping and ECV Standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson 2016;18(1):58.
- Akçakaya M, Basha TA, Weingärtner S, Roujol S, Berg S, Nezafat R. Improved quantitative myocardial T2 mapping: Impact of the fitting model. Magnetic Resonance in Medicine 2015;74(1):93-105.
- Tao Q, van der Tol P, Berendsen FF, Paiman EHM, Lamb HJ, van der Geest RJ. Robust motion correction for myocardial T1 and extracellular volume mapping by principle component analysis-based groupwise image registration. J Magn Reson Imaging 2018;47(5):1397-1405.
- Zhao Y, Yang C, Schweidtmann A, Tao Q. Efficient Bayesian Uncertainty Estimation for nnU-Net. In: Wang L, Dou Q, Fletcher PT, Speidel S, Li S, editors2022 2022//; Cham. Springer Nature Switzerland. p 535-544.
Figures
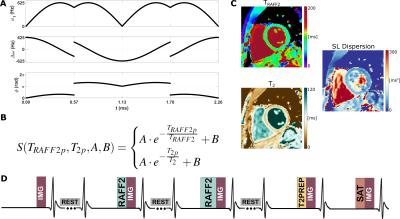
Figure 1: A) Single RAFF2 RF pulse block. Top: Amplitude modulation; Middle: Frequency-offset modulation; Bottom: Phase modulation. B) 4-parameter fit used to obtain simultaneous TRAFF2 and T2 maps. C) Approximate SL dispersion was obtained pixel-wise by normalizing the difference between the TRAFF2 and T2 maps with the RAFF2 pulse amplitude. D) Proposed approximate myocardial Spin-Lock (SL) dispersion mapping pulse sequence diagram: 5 bSSFP images with different preparations are acquired in a breath-hold. 4s rest periods are played to allow for magnetization recovery.
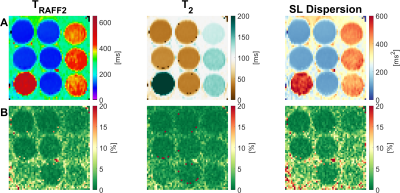
Figure 2: A) TRAFF2 (left column), T2 (middle column), and SL dispersion map (right column) acquired in the T1MES phantom. B) Corresponding coefficient of variation (CoV) map across the three repetitions. Excellent intra-scanner repeatability was achieved with CoV lower than 5% for all the vials.

Figure 3: Representative TRAFF2, T2, and approximate SL dispersion maps acquired in different healthy subjects, showing a clear depiction of the myocardium with a homogeneous signal in most of the cases. Residual B0-related artifacts were observed in some cases.
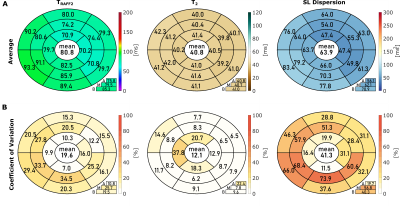
Figure 4: A) Bullseye plot with average TRAFF2 (left column), T2 (middle column), and approximate SL dispersion (right column) across all the measurements in the healthy subjects. B) Bullseye plot with the corresponding coefficient of variation (CoV) across the three repetitions for each subject. Segmentation was performed according to the AHA 16-segment model in three short-axis slices (A = apical, M = mid-ventricular, B = basal). The average across all segments is given in the center of the bullseye, the slice averages can be found below.
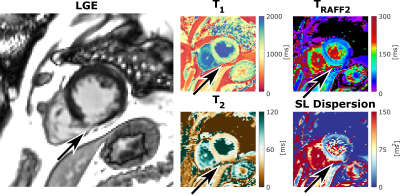
Figure 5: Short-axis (SAX) view of late gadolinium enhancement (LGE), native T1 map, as well as simultaneous TRAFF2, T2, and the corresponding approximate SL dispersion map, acquired in a sixty-five-year-old patient diagnosed with mid-right coronary artery (RCA) infarct. Scar tissue in the inferior segment (arrow) is visible in the LGE image, TRAFF2, and approximate SL dispersion map, while T1 and T2 present normal tissue.
DOI: https://doi.org/10.58530/2023/4456