4451
APT histogram parameters for Response Prediction of Neoadjuvant Chemotherapy in Rectal Cancer: A Prospective Study1Department of Radiology, West China Hospital, Sichuan University, Cheng du, China, 2Colorectal Cancer Center,Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China, 3Department of Radiology, West China Hospital, Sichuan University, Chengdu, China, 4Philips Healthcare, China, Beijing, China, 5Philips Healthcare, China, Chengdu, China
Synopsis
Keywords: Cancer, fMRI, rectal cancer
Early imaging prediction of neoadjuvant chemotherapy (NACT) response would enable a personalized treatment approach to improve therapeutic response and avoid treatment morbidity in rectal cancer. Morphological changes base on T2 weighted MRI and free dispersion limitation of water molecules in tumor have limited value in evaluating efficacy after NACT. Amide proton transfer (APT)–weighted MRI, indirectly detecting the concentration of tissue macromolecular proteins, could help to inform us of the proliferation and biological status of tumor cells. The aim of this study is to determine if APT MRI is useful in early assessment of treatment response in persons with rectal cancer.Synopsis
Early imaging prediction of neoadjuvant chemotherapy (NACT) response would enable a personalized treatment approach to improve therapeutic response and avoid treatment morbidity in rectal cancer. Morphological changes base on T2 weighted MRI and free dispersion limitation of water molecules in tumor have limited value in evaluating efficacy after NACT. Amide proton transfer (APT)–weighted MRI, indirectly detecting the concentration of tissue macromolecular proteins, could help to inform us of the proliferation and biological status of tumor cells. The aim of this study is to determine if APT MRI is useful in early assessment of treatment response in persons with rectal cancer.Summary of Main Findings
We found that APTkurtosis may be an effective biomarker to predict NACT response, and the APTkurtosis obtained after 1 cycle is expected to first provide accurate efficacy information.Introduction
Neoadjuvant chemotherapy (NACT) is recommended for low to moderate-risk rectal cancer (RC) before the total mesorectal excision [1,2], while only limited patients would achieve pathologic complete response (pCR) [3]. The indicators used to evaluate the NACT efficacy are mainly based on T2 morphological information, including the long diameter, thickness, and volume of the tumor [4,5], with a certain delay. In addition, diffusion weighted magnetic resonance imaging (DW-MRI), the only imaging technology for non-invasive detection of the restriction of free diffusion of water molecules in human body, is still limited in distinguishing between tumors and fibrosis after treatment [6]. Amide proton transfer (APT) imaging generates APT contrast via the amide protons located on the backbone of mobile proteins and peptides. APT MRI signal intensity increased in tumor cells which have an increased concentration of cytosolic mobile proteins owing to increased metabolic activities and cellular density [7]. Therefore, evaluating the protein content of tumor cells through APT imaging could help to inform us of the proliferation and biological status of tumor cells. Furthermore, histogram analysis may provide more information than the average alone [8]. However, whether APT can effectively distinguish the patients who are effective or not after neoadjuvant chemotherapy and when to promptly predict efficacy remains unclear. In this study, we prospectively and continuously investigated the APT values before, during and after NACT, aiming to determine the earliest time point and the most effective indicators for evaluation of NACT response in RC.Image Acquisition
MR protocol consisted of oblique axial T2-weighted sequence and APT sequence. MR images were collected at 5 time points (TPs), including TP1 (before treatment), TP2 (after 7 days), TP3 (after 1 cycle), TP4 (after 2 cycles) and TP5 (before operation), in all patients respectively. Regions of interest (ROI) of T2 images were manually placed on the tumor region, avoiding the necrotic, cystic and hemorrhagic regions in the whole tumor and were registered to APT maps to construct the APT histogram, including the mean, skewness and kurtosis [8].Statistics Analysis
APT histogram parameters between two groups were compared. For parameters with significant differences between groups, the changes at different time points for visualization of APT parameters and receiver operating characteristic (ROC) curve performed. Multiple comparative analyses were carried out to compare the difference of parameters between time points. Comparison of the areas under the curve (AUC) was performed.Results
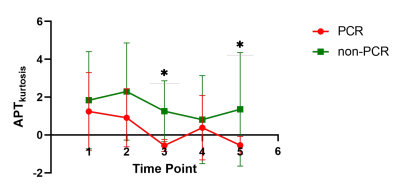
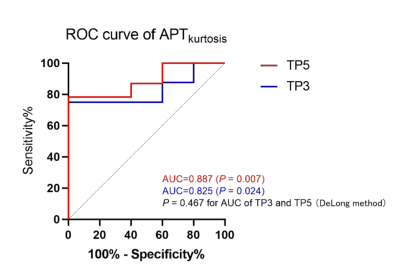
Between June 2021 and October 2022, 29 consecutive patients with biopsy-proven RC were recruited. The APTkurtosis of TP3 and TP5 were lower in pCR group (0.56±0.32 and 0,54±0.47, respectively) than in non-pCR group (0.89±0.57 and 1.32±1.58, respectively) (P values = 0.023 and 0.005, respectively; Fig 1). However, there were no diferences in other APT parameters among the 5 TPs between the two groups. The cutoff values, sensitivity, specificity and area under curve (AUC) of the APTkurtosis at TP3 and TP5 were -1.035, 100%, 80% and 0.825 (P =0.024), and -1.030, 100%, 80% and 0.887 (P =0.007) (P =0.467 for AUC of TP3 vs TP5; Fig. 2).Discussion
Our study demonstrated that APTkurtosis may contribute to predicting response in RC after 1 cycle of NACT and before operation with similar AUC values, and 1 cycle after NACT may be the earliest time for predicting pCR. Few studies evaluated the efficacy of neoadjuvant therapy by using APT values only before treatment or surgery [9,10,11] or evaluated the histopathological factors related to prognosis in RC without any neoadjuvant therapy before surgery [12,13,14,15]. Due to the limited sample size, our study was unable to evaluate the value of APT in evaluating the rectal fibrosis after treatment like Martens et al. [9]. Additionally, we found no difference between the pCR and non-PCR groups in the mean, kurtosis and skewness of APT values before treatment, which was different from the study of Chen al. [11]. More studies are needed to explore and verify the value of APT in efficacy prediction of NACT in RC. Our limitations lie in the relatively small sample size and some patients had small lesions. In addition, we didn't compare APT parameters with ADC or other parameters, which needs further exploration in future research.Conclusion
APT imaging is a promising noninvasive technique for monitoring and predicting therapeutic response in rectal cancer patients undergoing NACT and APTkurtosis after one cycle may be recommended to early predict the NACT efficacy in the future.Acknowledgements
No acknowledgement found.References
[1] Glynne-Jones, R., Wyrwicz, L., Tiret, E., Brown, G., Rödel, C., Cervantes, A., Arnold, D., & ESMO Guidelines Committee (2017). Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology, 28(suppl_4), iv22–iv40. https://doi.org/10.1093/annonc/mdx224
[2] Shiraishi, T., Sasaki, T., Ikeda, K., Tsukada, Y., Nishizawa, Y., & Ito, M. (2019). Predicting prognosis according to preoperative chemotherapy response in patients with locally advanced lower rectal cancer. BMC cancer, 19(1), 1222. https://doi.org/10.1186/s12885-019-6424-4
[3] M. Maas, P.J. Nelemans, V. Valentini, et al., Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data, Lancet Oncol. 11 (2010) 835–844, https://doi. org/10.1016/s1470-2045(10)70172-8.
[4] Deng, X., Wu, Q., Bi, L., Yu, Y., Huang, S., He, D., Wu, B., Gou, H., Meng, W., Qiu, M., He, Y., & Wang, Z. (2021). Early response to upfront neoadjuvant chemotherapy (CAPOX) alone in low- and intermediate-risk rectal cancer: a single-arm phase II trial. The British journal of surgery, 109(1), 121–128. https://doi.org/10.1093/bjs/znab388
[5] Ha, H. I., Kim, A. Y., Yu, C. S., Park, S. H., & Ha, H. K. (2013). Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. European radiology, 23(12), 3345–3353. https://doi.org/10.1007/s00330-013-2936-5
[6] Beets-Tan, R. G., & Beets, G. L. (2014). MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nature reviews. Gastroenterology & hepatology, 11(8), 480–488. https://doi.org/10.1038/nrgastro.2014.41
[7] Zhou, J., Payen, J. F., Wilson, D. A., Traystman, R. J., & van Zijl, P. C. (2003). Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature medicine, 9(8), 1085–1090. https://doi.org/10.1038/nm907
[8] Enkhbaatar, N. E., Inoue, S., Yamamuro, H., Kawada, S., Miyaoka, M., Nakamura, N., Sadahiro, S., & Imai, Y. (2018). MR Imaging with Apparent Diffusion Coefficient Histogram Analysis: Evaluation of Locally Advanced Rectal Cancer after Chemotherapy and Radiation Therapy. Radiology, 288(1), 129–137. https://doi.org/10.1148/radiol.2018171804
[9] Martens, M. H., Lambregts, D. M., Papanikolaou, N., Heijnen, L. A., Riedl, R. G., zur Hausen, A., Maas, M., Beets, G. L., & Beets-Tan, R. G. (2014). Magnetization transfer ratio: a potential biomarker for the assessment of postradiation fibrosis in patients with rectal cancer. Investigative radiology, 49(1), 29–34. https://doi.org/10.1097/RLI.0b013e3182a3459b
[10] Martens, M. H., Lambregts, D. M., Papanikolaou, N., Heijnen, L. A., Riedl, R. G., zur Hausen, A., Maas, M., Beets, G. L., & Beets-Tan, R. G. (2014). Magnetization transfer ratio: a potential biomarker for the assessment of postradiation fibrosis in patients with rectal cancer. Investigative radiology, 49(1), 29–34. https://doi.org/10.1097/RLI.0b013e3182a3459b
[11] Chen, W., Mao, L., Li, L., Wei, Q., Hu, S., Ye, Y., Feng, J., Liu, B., & Liu, X. (2021). Predicting Treatment Response of Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer Using Amide Proton Transfer MRI Combined With Diffusion-Weighted Imaging. Frontiers in oncology, 11, 698427. https://doi.org/10.3389/fonc.2021.698427
[12] Li, J., Lin, L., Gao, X., Li, S., & Cheng, J. (2022). Amide Proton Transfer Weighted and Intravoxel Incoherent Motion Imaging in Evaluation of Prognostic Factors for Rectal Adenocarcinoma. Frontiers in oncology, 11, 783544. https://doi.org/10.3389/fonc.2021.783544
[13] Nishie, A., Takayama, Y., Asayama, Y., Ishigami, K., Ushijima, Y., Okamoto, D., Fujita, N., Tsurumaru, D., Togao, O., Manabe, T., Oki, E., Kubo, Y., Hida, T., Hirahashi-Fujiwara, M., Keupp, J., & Honda, H. (2018). Amide proton transfer imaging can predict tumor grade in rectal cancer. Magnetic resonance imaging, 51, 96–103. https://doi.org/10.1016/j.mri.2018.04.017
[14] Li, L., Chen, W., Yan, Z., Feng, J., Hu, S., Liu, B., & Liu, X. (2020). Comparative Analysis of Amide Proton Transfer MRI and Diffusion-Weighted Imaging in Assessing p53 and Ki-67 Expression of Rectal Adenocarcinoma. Journal of magnetic resonance imaging : JMRI, 52(5), 1487–1496. https://doi.org/10.1002/jmri.27212
[15] Chen, W., Li, L., Yan, Z., Hu, S., Feng, J., Liu, G., Liu, B., & Liu, X. (2021). Three-dimension amide proton transfer MRI of rectal adenocarcinoma: correlation with pathologic prognostic factors and comparison with diffusion kurtosis imaging. European radiology, 31(5), 3286–3296. https://doi.org/10.1007/s00330-020-07397-1