4445
Role of tomoelastography for the differentiation between solid pseudopapillary neoplasms and pancreatic neuroendocrine neoplasms1The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, 2The First Affiliated Hospital, Sun Yat-Sen University, Guanghzou, China
Synopsis
Keywords: Pancreas, MR Value, Magnetic resonance elastographies,SPNs,pNENs
This prospective study enrolled patients with pathologically confirmed solid pseudopapillary tumors (SPNs) and pancreatic neuroendocrine tumors (pNENs) who underwent preoperative tomoelastography examinations at our hospital. Sixty-four patients were included (18 patients with SPNs; 46 patients with pNENs). SPNs showed significantly lower stiffness (P<0.001) and fluidity (P=0.001) than pNENs. Both stiffness and fluidity allowed distinguishing SPNs from pNENs. AUCs were 0.899 for stiffness, 0.760 for fluidity. Tomoelastography is a novel multi-frequency MRE technique that can facilitate the differentiation between SPNs and pNENs.Introduction
Solid pseudopapillary tumors (SPNs) of the pancreas are low-grade malignant pancreatic tumors (1), while pancreatic neuroendocrine tumors (pNENs) are a class of malignant heterogeneous pancreatic tumors with various manifestations (2). The early clinical features and imaging manifestations of pNENs are similar to SPNs, but the treatment strategies and prognosis of SPNs and pNENs are completely different. Surgery is the only treatment method for SPNs, but for pNENs, it can be selected different methods according to their function, tumor size and metastasis (3). Therefore, it is important for clinician to distinguish between these two diseases. Although endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) can possibly obtain a pathological diagnosis, its use was limited due to the experience of diagnostic physicians, the difference of sampling sites, and the characteristic of invasiveness (1, 4, 5).The changes of cell adhesion and fibrosis can change the mechanical properties of pancreatic tissues, providing a biological basis for the identification of both diseases. Tomoelastography is a multi-frequency magnetic resonance elastography (MRE) technique with noise-robust data post-processing, which can provide high-resolution parameter maps and quantify tissue stiffness and fluidity. Quantifying stiffness is helpful to evaluate fibrosis in tissues (6), and fluidity reflects the internal mechanical friction of biological tissue (7).
Therefore, in this prospective study, we investigated the stiffness and fluidity of pNENs and SPNs using tomoelastography and determined its diagnostic performance in differentiating between these two diseases.
Materials and Methods
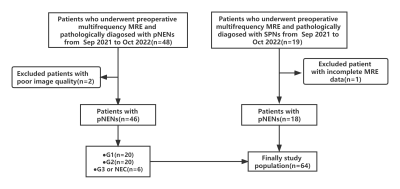
This prospective single-center study was approved by the institutional review board of our hospital and the written informed consent were received from patients. The flow chart was shown in Figure 1. According to the WHO 2019 classification criteria, pNENs lesions were graded and staged into four categories: neuroendocrine tumour-G1 (NET-G1), neuroendocrine tumour-G2 (NET-G2), neuroendocrine tumour-G3 (NET-G3), and neuroendocrine carcinoma-G3 (NEC-G3). The entire pancreatic tomoelastography was scanned in 7 minutes and 22 seconds covered by 35 contiguous axial sections. The multifrequency wave field data was processed at https://bioqic-apps.com. The region of interests (ROIs) were placed at the lesions, avoiding boundary and artifacts, to measure stiffness (on c map) and fluidity (on φ map), as the Figure 2 shown.Results
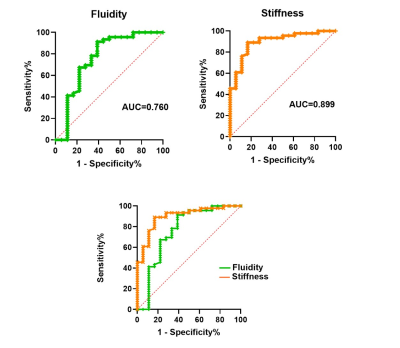
A total of 64 patients were enrolled in this study. There were 18 patients pathologically diagosed with SPNs and 46 with pNENs. Among the patients with pNENs, there were 20 graded and staged with G1, 20 with G2 and 6 with G3 or NEC. There was significant difference between the lesion of pNENs and SPNs in stiffness (P<0.001) and fluidity (P=0.001). The use of c enabled to differentiate patients between pNENs and SPNs with an AUC of 0.889 (cutoff, 1.897 m/sec), and the use of φ with an AUC of 0.760 (cutoff, 0.906 radian), as the Figure 3 shown.Discussion
From the perspective of precision medicine and the different prognosis and surgical strategies, there is still a clinical need for straightforward and non-invasive modality to differentiate patients between SPNs and pNENs. In our study, SPNs showed significantly lower stiffness (P<0.001) and fluidity (P=0.001) than pNENs. Both stiffness and fluidity allowed distinguishing SPNs from pNENs, and AUCs were 0.899 for stiffness, 0.760 for fluidity. . In conclusion, tomoelastography is a novel and promising tool to distinguish SPNs from pNENs.Acknowledgements
The authors sincerely acknowledge Ms. Jing Guo from Department of Radiology of Charité –Universitätsmedizin Berlin, Germany and Mengzhu Wang from MR Scientific Marketing, Siemens Healthineers Ltd. Guangzhou, China for the MR technical support.References
1. Abdelkader A, Hunt B, Hartley CP, Panarelli NC, Giorgadze T. Cystic Lesions of the Pancreas: Differential Diagnosis and Cytologic-Histologic Correlation. Arch Pathol Lab Med. 2020;144(1):47-61. doi:10.5858/arpa.2019-0308-RA
2. Ma ZY, Gong YF, Zhuang HK, et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol. 2020;26(19):2305-2322. doi:10.3748/wjg.v26.i19.2305
3. Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844-860. doi:10.1016/j.annonc.2020.03.304
4. Liu Y, Shi S, Hua J, et al. Differentiation of solid-pseudopapillary tumors of the pancreas from pancreatic neuroendocrine tumors by using endoscopic ultrasound. Clin Res Hepatol Gastroenterol. 2020;44(6):947-953. doi:10.1016/j.clinre.2020.02.002
5. Krishna SG, Bhattacharya A, Li F, et al. Diagnostic Differentiation of Pancreatic Neuroendocrine Tumor From Other Neoplastic Solid Pancreatic Lesions During Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Pancreas. 2016;45(3):394-400. doi:10.1097/MPA.0000000000000488
6. Venkatesh SK, Wells ML, Miller FH, et al. Magnetic resonance elastography: beyond liver fibrosis-a case-based pictorial review. Abdom Radiol (NY). 2018;43(7):1590-1611. doi:10.1007/s00261-017-1383-1
7. Streitberger KJ, Lilaj L, Schrank F, et al. How tissue fluidity influences brain tumor progression. Proc Natl Acad Sci U S A. 2020;117(1):128-134. doi:10.1073/pnas.1913511116
Figures