4440
The PRISM clinical trial: Quantitative imaging for personalized liver radiation therapy1Institute of Medical Physics, The University of Sydney, Sydney, Australia, 2Sydney West Radiation Oncology Network, Western Sydney Local Health District, Westmead, Australia, 3NHMRC Clinical Trials Centre, The University of Sydney, Sydney, Australia, 4Faculty of Medicine and Health, The University of Sydney, Sydney, Australia, 5Department of Radiology, Westmead Hospital, Westmead, Australia, 6Acute Surgical Unit, Westmead Hospital, Westmead, Australia, 7Ingham Institute of Applied Medical Research, Liverpool, Australia, 8Northern Sydney Cancer Centre, Royal North Shore Hospital, St Leonards, Australia, 9Department of Gastroenterology and Hepatology, Westmead Hospital, Westmead, Australia, 10Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom
Synopsis
Keywords: Cancer, Radiotherapy
Quantitative imaging biomarkers of liver function offer a potential opportunity to optimize radiation dose distributions for personalized treatment of patients with liver cancers using radiation therapy. The PRISM clinical study aims to determine if radiation treatment plans guided by liver function maps from MRI can increase the therapeutic dose to tumours. We have developed a dedicated multi-parametric MRI protocol for the quantitative assessment of liver composition, structure and function. Details of the protocol and pilot data are presented here.
Introduction
Stereotactic body radiation therapy (SBRT) is an emerging treatment option for patients with hepatocellular carcinoma (HCC). Baseline liver function, typically described by the Child-Pugh (CP) score determines dose constraints to the non-tumour liver, in turn limiting the dose delivered to the gross tumour volume (GTV)1. Therefore, for many patients with impaired liver function, SBRT is offered as a palliative option. However, patients with HCC can have a high degree of spatial variation in liver function due to underlying chronic liver disease. The personalised liver SBRT using magnetic resonance imaging (PRISM) clinical study aims to determine if using quantitative liver function maps derived from MRI for SBRT planning can increase the radiation dose that can be safely delivered to the GTV.Here, we present the multi-parametric MRI protocol for quantitative mapping of liver function for the PRISM clinical study, along with pilot data on a healthy volunteer subject, obtained as part of protocol development.
Methods
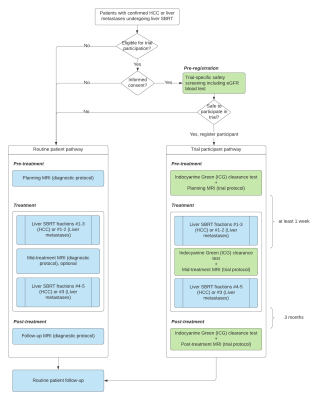
PRISM study designIn this prospective study (ACTRN12622000371796, www.anzctr.org.au), a total of 30 patients with HCC or liver metastases eligible for liver SBRT will be recruited. The number of participants with liver metastases will be limited to <1/3rd of study participants to include good and impaired baseline liver function. Study participants will undergo pre-, mid-, and 3-month post-treatment MRI scans. An indocyanine green (ICG) clearance test2 will be performed prior to each MRI scan as a surrogate measure of global liver function. The study schema is shown in Figure 1.
The study will be performed in three stages – Stage 1 will gather preliminary data from 5 participants to optimise the study design, including the MRI scan and ICG test protocol; Stage 2 will evaluate the potential of MRI to distinguish between high and low functioning regions of the liver and spatially define changes in liver function as a response to radiation dose on a further 10 patients; in Stage 3, data will be collected for a further 15 participants. Quantitative liver function maps derived from the MR images will be used to create PRISM SBRT plans where radiation dose to high functioning regions of the liver will be minimized, and low functioning liver sacrificed to maximize the dose delivered to the GTV. Mid-treatment liver function maps will be used to adapt PRISM SBRT plans to further optimize dose distributions.
Results
PRISM study MRI protocolA multi-parametric MRI protocol was iteratively developed on a MAGNETOM Prisma 3T MRI scanner (Siemens Healthcare GmbH, Erlangen, Germany). The protocol consists of 2 localisers, acquired with the subject in breath-hold and free breathing; a whole-liver transverse T2w-HASTE with fat suppression for anatomical reference using an expiration breath-hold scan for co-registration with SBRT planning CT scans. Transverse T1w-DIXON sequences were included for fat-water imaging and inline proton density fat fraction (PDFF) and R2* evaluation, followed by DWI with 13 b-values (0, 10, 20, 30, 40, 50, 60, 70, 80, 100, 200, 400, 800 s/mm2) for IVIM modelling for estimation of the slow diffusion coefficient (D) and perfusion fraction (f). T1 mapping was included with variable flip angles (VFA, FA = 6.5, 15, 27°) and B1 mapping for field inhomogeneity correction, followed by DCE-MRI using a 3D radial stack-of-stars sequence3 acquired in free breathing.
Pilot data
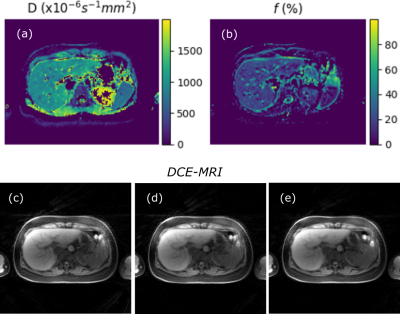
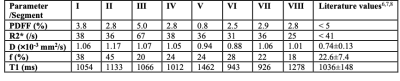
Data acquired from a healthy volunteer subject is presented here. IVIM modelling was implemented using custom-written Python code. DCE-MRI was acquired without contrast agent injection, and retrospectively reconstructed to a temporal resolution of 7 seconds using open-source, dedicated MATLAB code for offline reconstruction4 (https://bitbucket.org/yarra-dev/yarramodules-grasp-basic). Example IVIM parametric maps (diffusion coefficient D, perfusion fraction f) and DCE-MRI frames (t=0, 7 and 14 sec) are shown in Figure 2. Circular regions of interest (ROI) were manually selected in individual liver segments5 and mean values of the quantitative parameters were obtained using ITK-SNAP6. Measured PDFF, R2*, f, D and T1 were found to be comparable to literature values in the healthy liver as shown in Table 1, although measurements were found to vary across the liver segments.
Outlook
Preliminary results demonstrate feasibility of a multi-parametric liver MRI protocol for quantitative mapping of liver structure and function. The PRISM clinical trial has received ethics approval (WSLHD-HREC: 2022/ETH00203) and patient recruitment will commence in November 2022. Results from Stage 1 of the study are expected by mid-2023.Acknowledgements
We acknowledge the Sydney West Radiation Oncology Network for supporting this work and funding Stage 1 of the study. We thank A/Prof. Tobias Block, Dr. Jonathan Goodwin, Ms. Kate Skehan, Ms. Yu-Feng Wang and Siemens Healthineers for their support on development of the MRI protocol, and Prof. Geoff Parker on valuable discussions on this study.
References
- Liu H, Lee Y-Y, Sridharan S et al. Stereotactic body radiotherapy in the management of hepatocellular carcinoma. JMIRO. 2021;65(3):365–373.
- Suresh K, Owen D, Bazzi L et al. Using Indocyanine Green Extraction to Predict Liver Function. Int J Radiat Oncol. 2018;100(1):131–137.
- Block KT, Chandarana H, Milla S et al. Towards routine clinical use of radial stack-of-stars 3D gradient-echo sequences for reducing motion sensitivity. J Korean Soc Magn Reson Med. 2014;18(2):87-106.
- Feng L, Grimm R, Block KT et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72(3):707-717.
- Germain T, Favelier S, Cercueil JP et al. Liver segmentation: Practical tips. Diagn Interv Imaging. 2014;95(11):1003-1016.
- Yushkevich PA, Piven J, Hazlett HC et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116-1128.
- Kuhn J-P, Meffert P, Heske C et al. Prevalence of fatty liver disease and hepatic iron overload in a Northeastern German population by using quantitative MR imaging. Radiology. 2017;284(3):706-716.
- Wurnig MC, Donati OF, Ulbrich E et al. Systematic analysis of the intravoxel incoherent motion threshold separating perfusion and diffusion effects: Proposal of a standardized algorithm. Magn Reson Med. 2015;74(5):1414-1422.
- Tadimalla S, Wilson DJ, Shelley D et al. Bias, repeatability and reproducibility of liver T1 mapping with variable flip angles. JMRI. 2022;56(4)1042-1052.
Figures