4438
Preoperative Prediction of Hepatocellular Carcinoma with Stem Cell Phenotype on Enhanced Magnetic Resonance Imaging and Its Prognostic Value1Radiology, West China Hospital, Sichuan University, Chengdu, China
Synopsis
Keywords: Liver, Tumor
Hepatocellular carcinoma (HCC) with stem cell phenotype is characterized by activating classic cell proliferation pathways and altering intrinsic regulators, it can stimulate HCC tumor proliferation, make the tumor more prone to recurrence, metastasis and therapy resistance. In this study, we developed an easy-to-use and noninvasive risk score integrating preoperative clinical indicators and enhancement magnetic resonance imaging (MRI) to predict stem cell phenotype HCC.Abstract
Introduction: Hepatocellular carcinoma (HCC) with stem cell phenotype is characterized by activating classic cell proliferation pathways and altering intrinsic regulators, it can stimulate HCC tumor proliferation, make the tumor more prone to recurrence, metastasis and therapy resistance. However, its evaluation still relies on invasive histopathologic examination. Therefore, we aimed to develop an easy-to-use and noninvasive risk score integrating preoperative clinical indicators and enhancement magnetic resonance imaging (MRI) to predict stem cell phenotype HCC.Methods: This retrospective study included consecutive patients with hepatectomy and pathological confirmed solitary HCC who underwent preoperative enhancement MRI between January 2010 and May 2021. MRI features were independently evaluated by two abdominal radiologists and the stem cell phenotype HCC was determined by two liver pathologists. For predictive the stem cell phenotype HCC, a scoring system was developed on the training dataset via logistical regression analysis. Model performances were assessed by computing areas under the curve (AUCs) of receiver operating characteristic (ROC). Survival outcomes were evaluated using the Kaplan-Meier method and compared with the log-rank test.
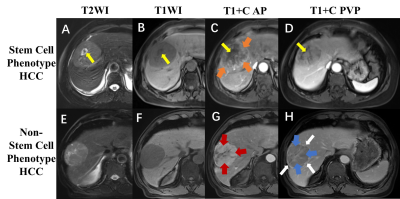
Results: A total of 334 patients (training set, n=232; independent external validation set, n=83) with solitary HCC (138 with stem cell phenotype positive HCC) were included in this study. Four enhancement MRI features were assigned into the stem cell phenotype HCC predictive model (with a scoring system) which including “rim arterial phase hyperenhancement (APHE)” (OR=5.9, 10 points), “nodule in nodule architecture” (OR=3.5, 7 points), “non-smooth tumor margin” (OR=1.6, 3 points) and “nonperipheral washout” (OR=0.6, -3 points). The optimal threshold of scoring system was 5.5 points, with AUC 0.75 and 0.73 on the training set and independent external validation sets, respectively. Prognosis after hepatic resection was worse in stem cell phenotype HCC patients, the poorer recurrence-free survival and overall survival were detected in those patients with pathologically confirmed or model predicted stem cell phenotype HCC.
Conclusion: Based on the three significant enhancement MRI features, we developed an easy-to-use and noninvasive risk score model which could accurately predict stem cell phenotype HCC, and revealed the patients with pathologically confirmed or model predicted stem cell phenotype HCC have worse prognosis, which may help improve personalized treatment decision making.
Acknowledgements
This work would thanks to funding by the National Natural Science Foundation of China (Grant No. 82101997, 81971571) and the Science and Technology Department of Sichuan Province (Grant No. 2022YFS0071, 2021YFS0021, 2021YFS0141).References
1. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nature reviews Disease primers. 2021 Jan 21;7(1):6.
2. Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver international : official journal of the International Association for the Study of the Liver. 2022 Aug;42(9):2029-41.
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021 May;71(3):209-49.
4. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017 Mar;152(4):745-61.
5. Lee TK, Guan XY, Ma S. Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nature reviews Gastroenterology & hepatology. 2022 Jan;19(1):26-44.
6. Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015 Oct;149(5):1226-39.e4.
7. Network CGAR. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell. 2017 Jun 15;169(7):1327-41.e23.
8. Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Biomedicines. 2021 Nov 8;9(11).
9. Delire B, Stärkel P. The Ras/MAPK pathway and hepatocarcinoma: pathogenesis and therapeutic implications. European journal of clinical investigation. 2015 Jun;45(6):609-23.
10. Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signaling pathway in cancer therapy. Expert opinion on therapeutic targets. 2012 Jun;16(6):553-72.
11. Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nature reviews Cancer. 2012 Jan 24;12(2):89-103.
12. Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006 Jun 26;25(27):3818-22.
13. Roskams T. Different types of liver progenitor cells and their niches. Journal of hepatology. 2006 Jul;45(1):1-4.
14. Wendum D, Layese R, Ganne-Carrié N, Bourcier V, Merabtene F, Cagnot C, et al. Influence of Progenitor-Derived Regeneration Markers on Hepatitis C Virus-Related Cirrhosis Outcome (ANRS CO12 CirVir Cohort). Hepatology (Baltimore, Md). 2018 Oct;68(4):1534-48.
15. Choi SY, Kim SH, Park CK, Min JH, Lee JE, Choi YH, et al. Imaging Features of Gadoxetic Acid-enhanced and Diffusion-weighted MR Imaging for Identifying Cytokeratin 19-positive Hepatocellular Carcinoma: A Retrospective Observational Study. Radiology. 2018 Mar;286(3):897-908.
16. Chen J, Wu Z, Xia C, Jiang H, Liu X, Duan T, et al. Noninvasive prediction of HCC with progenitor phenotype based on gadoxetic acid-enhanced MRI. European radiology. 2020 Feb;30(2):1232-42.
17. Guo Y, Chen J, Zhang Y, Guo Y, Jiang M, Dai Y, et al. Differentiating Cytokeratin 19 expression of hepatocellular carcinoma by using multi-b-value diffusion-weighted MR imaging with mono-exponential, stretched exponential, intravoxel incoherent motion, diffusion kurtosis imaging and fractional order calculus models. European journal of radiology. 2022 May;150:110237.
18. Wang HQ, Yang C, Zeng MS, Rao SX, Ji Y, Weng X, et al. Magnetic resonance texture analysis for the identification of cytokeratin 19-positive hepatocellular carcinoma. European journal of radiology. 2019 Aug;117:164-70.
19. Wang W, Gu D, Wei J, Ding Y, Yang L, Zhu K, et al. A radiomics-based biomarker for cytokeratin 19 status of hepatocellular carcinoma with gadoxetic acid-enhanced MRI. European radiology. 2020 May;30(5):3004-14.
20. Geng Z, Zhang Y, Wang S, Li H, Zhang C, Yin S, et al. Radiomics Analysis of Susceptibility Weighted Imaging for Hepatocellular Carcinoma: Exploring the Correlation between Histopathology and Radiomics Features. Magnetic resonance in medical sciences : MRMS : an official journal of Japan Society of Magnetic Resonance in Medicine. 2021 Sep 1;20(3):253-63.
21. Yang F, Wan Y, Xu L, Wu Y, Shen X, Wang J, et al. MRI-Radiomics Prediction for Cytokeratin 19-Positive Hepatocellular Carcinoma: A Multicenter Study. Frontiers in oncology. 2021;11:672126.
Figures