4417
A Flexible iPRES AIR Coil Array for MRI and Localized B0 Shimming1Duke-UNC Brain Imaging & Analysis Center, Duke University, Durham, NC, United States, 2Duke Medical Physics Graduate Program, Duke University, Durham, NC, United States, 3GE Healthcare, Aurora, OH, United States
Synopsis
Keywords: Shims, RF Arrays & Systems, flexible coil array, localized B0 shimming, integrated RF/shim coil array, iPRES
Susceptibility-induced B0 inhomogeneities can cause artifacts that severely degrade the image quality in many applications. Integrated parallel reception, excitation, and shimming (iPRES) coil arrays can perform MRI and localized B0 shimming with a single coil array, but are so far rigid, which limits their SNR and shimming performance. Here, we develop a flexible iPRES coil array that can conform to the subject’s anatomy and substantially improve the SNR and reduce the B0 inhomogeneities and geometric distortions in diffusion-weighted imaging of the knee compared to a rigid iPRES coil array, which will be valuable for many anatomical regions and applications.Introduction
Susceptibility differences at air/tissue interfaces induce localized B0 inhomogeneities throughout the body, which can cause distortions, signal loss, and incomplete fat suppression, reducing the image quality in many applications. Integrated RF/shim coil arrays, also known as integrated parallel reception, excitation, and shimming (iPRES) coil arrays, allow RF and DC currents to flow on the same coil elements, enabling MRI and localized B0 shimming with a single coil array and minimal SNR penalty. They can effectively shim localized B0 inhomogeneities in the brain1–4, abdomen5, breast6, and spine7, but are so far rigid and cannot conform to the subject’s anatomy to achieve an optimal SNR and shimming performance.In contrast, flexible coil arrays8–10, such as adaptive image receive (AIR™) coil arrays11–12, can conform to different subjects and body parts, increasing the ease of positioning, comfort, SNR, and versatility. AIR coil elements also benefit from a wider range of critical overlaps12 and larger freedom of positioning to improve the SNR, and from a lack of lumped capacitors that would otherwise require many inductors to implement iPRES. Here, we integrate the iPRES and AIR coil technologies into a flexible and lightweight iPRES AIR coil array that can conform to the subject’s anatomy and perform MRI and localized B0 shimming with a high SNR and shimming performance.
Methods
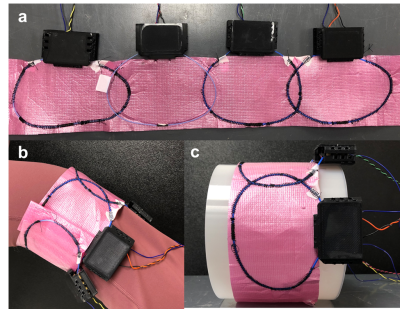
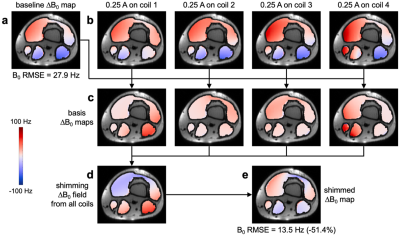
A flexible coil array was constructed with four 12x9-cm AIR coil elements with a 2-cm overlap (Figure 1a). DC wires and chokes were added between each coil element and a power supply to provide DC currents for shimming and to maintain the SNR. The coil elements were sewn into a polymer sheet that was tightly wrapped around the subject to prevent motion due to Lorentz forces induced by the switching of DC currents inside the scanner.MRI data were acquired on a 3T GE Premier UHP scanner in the lower thigh/upper knee of a healthy subject (Figure 1b). After linear shimming from the scanner, four ∆B0 maps were acquired with a multi-echo gradient-echo sequence (voxel size: 3x3x3 mm3, scan time: 29 s) with a DC current of 0.25 A separately applied in each coil element (Figure 2b). A baseline ∆B0 map acquired without DC currents (Figure 2a) was subtracted from each of them to yield basis ∆B0 maps representing the ∆B0 field generated by each coil element (Figure 2c). The optimal DC currents to shim each slice were computed by minimizing the B0 root-mean-square error (RMSE) between the baseline ∆B0 map and a combination of basis ∆B0 maps, within a mask excluding fat and bone regions. Another ∆B0 map was acquired with these optimal DC currents (Figure 2e).
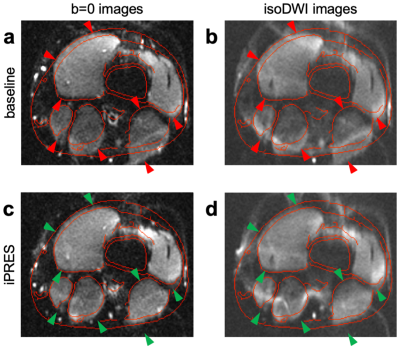
Spin-echo single-shot EPI images (TE: 55 ms, voxel size: 1x1x3 mm3) were acquired without or with diffusion-weighting (b-value: 600 s/mm2) and without or with the optimal DC currents. Undistorted fast spin-echo images were also acquired without DC currents to derive contour lines, which were overlaid on the EPI images.
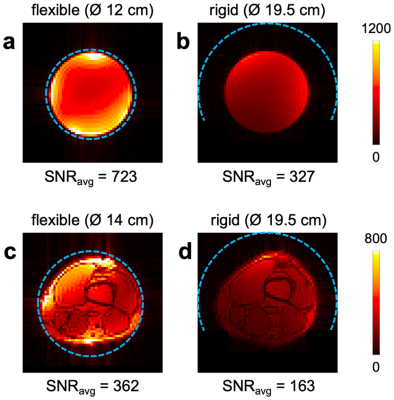
To demonstrate the improved SNR and shimming performance of the flexible iPRES AIR coil array relative to a rigid version, the coil array was also wrapped around a rigid tube with a 19.5-cm diameter to fit 99% of knee sizes9 (Figure 1c). SNR maps were acquired in a phantom and in the knee with the flexible coil array directly wrapped around them or with the rigid coil array. Shimming was also performed in the knee with the rigid coil array.
Results
The baseline ∆B0 map shows strong localized B0 inhomogeneities throughout the imaged region (Figure 2a). The flexible iPRES AIR coil array could generate a localized ∆B0 field in all quadrants (Figure 2c), which, when weighted by the optimal DC currents (0.26, -0.09, -0.37, and 0.08 A) and combined (Figure 2d), could substantially reduce the B0 inhomogeneities in the subject (Figure 2e), resulting in a 51.4% reduction in B0 RMSE.The baseline EPI images show severe distortions relative to the undistorted contour lines (Figure 3a,b; red arrowheads), which were drastically reduced after shimming with the flexible iPRES AIR coil array (Figure 3c,d; green arrowheads).
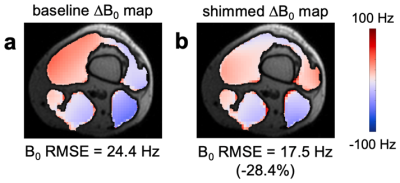
As expected, the rigid iPRES AIR coil array had a substantially lower SNR (Figure 4) and shimming performance (Figure 5; 28.4% reduction in B0 RMSE) than the flexible iPRES AIR coil array because the coil elements were further away and covered only approximately half rather than the whole circumference of the phantom/knee.
Discussion & Conclusion
The flexible iPRES AIR coil array can conform to the subject’s anatomy and perform MRI and localized B0 shimming with a single coil array, resulting in a greatly improved ease of positioning, comfort, SNR, and shimming performance compared to rigid iPRES coil arrays, which will be valuable for many anatomical regions and applications.Larger flexible iPRES AIR coil arrays with more coil elements could further improve the shimming performance and anatomical coverage. Unlike rigid iPRES coil arrays, for which basis ∆B0 maps are acquired only once in a phantom, flexible iPRES AIR coil arrays require them to be acquired in vivo for each subject, which may be impractical for many coil elements. However, more efficient approaches have been proposed to mitigate this issue13.
Acknowledgements
This work was in part supported by GE Healthcare and grants R01 EB028644, R01 NS075017, S10 OD021480, and S10 OD028544 from the National Institutes of Health.References
1. Truong, T.-K., Darnell, D., Song, A.W., 2014. Integrated RF/shim coil array for parallel reception and localized B0 shimming in the human brain. NeuroImage 103, 235–240. doi:10.1016/j.neuroimage.2014.09.052
2. Stockmann, J.P., Witzel, T., Keil, B., Polimeni, J.R., Mareyam, A., Lapierre, C., Setsompop, K., Wald, L.L., 2016. A 32-channel combined RF and B0 shim array for 3T brain imaging. Magnetic Resonance in Medicine 75, 441–451. doi:10.1002/mrm.25587
3. Willey, D., Darnell, D., Song, A.W., Truong, T.-K., 2021. Application of an integrated radio‐frequency/shim coil technology for signal recovery in fMRI. Magnetic Resonance in Medicine 86, 3067–3081. doi:10.1002/mrm.28925
4. Stockmann, J.P., Arango, N.S., Witzel, T., Mareyam, A., Sappo, C., Zhou, J., Jenkins, L., Craven‐Brightman, L., Milshteyn, E., Davids, M., Hoge, W.S., Sliwiak, M., Nasr, S., Keil, B., Adalsteinsson, E., Guerin, B., White, J.K., Setsompop, K., Polimeni, J.R., Wald, L.L., 2022. A 31‐channel integrated “AC/DC” B0 shim and radiofrequency receive array coil for improved 7T MRI. Magnetic Resonance in Medicine 87, 1074–1092. doi:10.1002/mrm.29022
5. Darnell, D., Truong, T.-K., Song, A.W., 2017. Integrated parallel reception, excitation, and shimming (iPRES) with multiple shim loops per radio‐frequency coil element for improved B0 shimming. Magnetic Resonance in Medicine 77, 2077–2086. doi:10.1002/mrm.26267
6. Ma, Y., Darnell D., Zhang H., Robb F., Song A.W., Truong TK. 2018. Integrated parallel reception, excitation, and shimming (iPRES) breast coil array for simultaneous MR image acquisition and localized B0 shimming. Proc. Intl. Soc. Mag. Reson. Med. 26, 842.
7. Cuthbertson, J.D., Truong, T.-K., Stormont, R., Robb, F., Song, A.W., Darnell, D., 2022. An iPRES‐W coil array for simultaneous imaging and wireless localized B0 shimming of the cervical spinal cord. Magnetic Resonance in Medicine 88, 1002–1014. doi:10.1002/mrm.29257
8. Frass-Kriegl, R., Navarro De Lara, L.I., Pichler, M., Sieg, J., Moser, E., Windischberger, C., Laistler, E., 2018. Flexible 23-channel coil array for high-resolution magnetic resonance imaging at 3 Tesla. PLOS ONE 13, e0206963. doi:10.1371/journal.pone.0206963
9. Zhang, B., Brown, R., Cloos, M., Lattanzi, R., Sodickson, D., Wiggins, G., 2019. Size-adaptable “Trellis” structure for tailored MRI coil arrays. Magnetic Resonance in Medicine 81, 3406–3415. doi:10.1002/mrm.27637
10. Yeh, J.-N.T., Lin, J.-F.L., Li, Y.-T., Lin, F.-H., 2019. A Flexible and Modular Receiver Coil Array for Magnetic Resonance Imaging. IEEE Transactions on Medical Imaging 38, 824–833. doi:10.1109/tmi.2018.2873317
11. McGee, K.P., Stormont, R.S., Lindsay, S.A., Taracila, V., Savitskij, D., Robb, F., Witte, R.J., Kaufmann, T.J., Huston, J., Riederer, S.J., Borisch, E.A., Rossman, P.J., 2018. Characterization and evaluation of a flexible MRI receive coil array for radiation therapy MR treatment planning using highly decoupled RF circuits. Physics in Medicine & Biology 63, 08NT02. doi:10.1088/1361-6560/aab691
12. Collick, B. D., et al., 2020. Rapid development of application-specific flexible MRI receive coils. Physics in Medicine & Biology 65, 19NT01.
13. Arango, N., White, J., Adalsteinsson, E., 2022. A fast magnetostatic inverse approach for subject-specific ∆B0 shim coil calibration. Proc. Intl. Mag. Reson. Med. 30, 1374.
Figures