4415
Optimized interferometric encoding of presaturated TurboFLASH B1-mapping for pTx at 7T MRI: Application to T1-mapping in the spinal cord1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3iLab-Spine, International Associated Laboratory, Montréal, Canada, Marseille, France, 4Paris-Saclay University, CEA, CNRS, BAOBAB, NeuroSpin, Gif-sur-yvette, France, 5Siemens Healthcare SAS, Saint-Denis, France
Synopsis
Keywords: RF Pulse Design & Fields, Spinal Cord
One of the best solutions to B1 inhomogeneities observed at ultra-high field MRI is to use parallel transmit techniques (pTx). However, fast and accurate knowledge of the B1+ is required. The presaturated TurboFLASH (satTFL)-based B1-mapping sequence, with interferometric encoding, has shown great promise due to its speed and reliability, but it was observed that its performance may drop when used with unconventional radiofrequency coils and applications. In this study, a novel optimization of the interferometric encoding is presented and applied to cervical spinal cord MRI at 7T. The impact of having improved B1-maps is evaluated on pTx MP2RAGE-based T1-mapping.Introduction
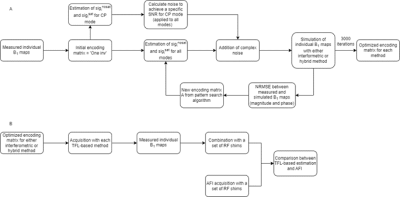
Parallel transmit (pTx) techniques are promising solutions to mitigate B1-related issues1–3. As they require B1 generated by each channel, having accurate B1-maps is primordial. Several B1-mapping sequences have been developed, with varying results4, with one of the most accurate being the actual flip angle (AFI) sequence, although it is prohibitively slow for mapping multiple RF channels5. Alternatively, the presaturated TurboFLASH (satTFL) method with interferometry encoding can provide fast individual B1-maps4 from acquired linear combinations B1lc of RF channels:$$$ B_{1 ,i}^{lc}=∑_k A_{i,k} B_{1,k} $$$ and $$$ B_{1 ,k}=∑_i (A^{-1} )_{i,k} B_{1,k}^{lc} $$$
where $$$ i $$$ is the i-th RF mode, $$$ k $$$ is the k-th RF-channel, and $$$ A$$$ is the interferometry encoding matrix. Simple $$$A$$$-matrices are used, such as ‘One-inv’ (one channel has opposite phase than others)6, and optimization of diagonal elements of $$$ A$$$ was proposed7. In this work, we evaluate the accuracy of the satTFL for cervical spinal cord compared with the AFI and apply a novel optimization of the interferometric encoding, as it was observed that B1-inhomogeneities resulted in signal drops over all B1lc with the used RF coil. The influence of the optimization was evaluated on in vivo quantitative T1-mapping from MP2RAGE with pTx .
Methods
B1-mapping was performed at 7T (TERRA, Siemens Healthcare, Germany), with a Rapid 8Tx/8Rx cervical spine coil loaded with the SAM phantom (Speag, Switzerland)8, and with two volunteers (Fig.3).SatTFL was used to measure 2D-axial individual B1-maps with interferometric acquisitions from a modified version using a Shinnar-Le Roux saturation pulse9 with two methods:
- Interferometric10: Eight RF modes i, with and without presaturation-pulse; effective flip angle $$$β_{i}^{lc} = acos(sig_i^{sat}/sig_i^{nosat}).$$$
- Hybrid11,12: Eight RF modes i, without presaturation pulse and a single reference B1-map, with $$$β_{i}^{lc} = β_{ref}.(sig_i^{nosat}/sig_{ref}^{nosat})$$$ and $$$β_{ref}^{lc} = acos(sig_{ref}^{sat}/sig_{ref}^{nosat}).$$$
- Effective excitation and presaturation FA, α and β, were calculated by numerical integration of the Bloch equation using the measured B1.
- $$$ sin_{i}^{nosat} = sin(α) + n_1 $$$ and $$$ sin_{i}^{sat} = sin(α).cos(β) + n_2 $$$ , with $$$n_1$$$ and complex Rice noise scaled to obtain SNR = 15. The signal model could be simplified as the ratio of the two images is used.
To further evaluate the influence of the optimized satTFL, MP2RAGE-based T1-maps were finally acquired using pTx pulses calculated from the different hybrid satTFL13, and compared with standard acquisition with vendor RF pulses, and B1+-correction8.
Results
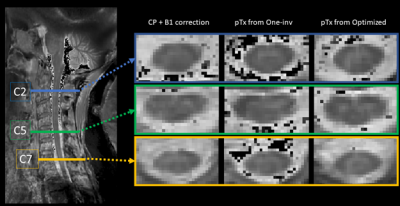
Fig.2 shows sagittal views of the calculated and measured B1-maps, and the contour of the ROI in which the B1 reliability was optimized. Results show a substantially better match with the AFI in the region of low FA after optimization, corresponding to lower cord levels (white arrows). This led to lower accuracy outside the ROI, however this signal is not used in the targeted applications.Fig.3 confirms those observations, showing a scatter plot of calculated B1 maps compared with the AFI. Although the One-inv and Optimized methods have similar accuracies around FA=50° to 60°, substantial improvement of the match is observed at lower FA (phantom) and higher FA (in vivo) after optimization.
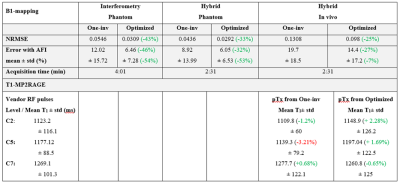
A quantification of this observation is shown in Table.1 (top), as the NMRSE and percentage errors compared with the AFI, showing that reduction of errors up to 46% was achieved. The hybrid Optimized method provided the best accuracy and fastest acquisition.
Fig.4 shows T1-maps at three cervical levels, showing greater GM/WM contrast with pTx pulses. Table.1 (bottom) shows that pTx MP2RAGE provides results within inter-session reproducibility without need to correct for B1-inhomogeneity, with more reliable results when using optimized satTFL.
Discussion and Conclusion
This study shows that although satTFL provides fast and accurate B1-maps, it deteriorates in some regions with the commonly used ‘One-inv’ (using the C-spine RF coil). This impacts the prediction of absolute B1-maps in low SNR regions when using pTx, which can be observed in quantitative T1 measures. Optimizing the amplitude and phase of the matrix $$$ A $$$ greatly improves accuracy inside ROI.Because of the large number of degrees-of-freedom (128 and 144 for Interferometry and Hybrid methods, respectively), the stopping criteria was reached in about 1h. Rather than a patient-specific optimization, the optimization of the matrix $$$ A $$$ should be calculated offline including a wide range of acquired in-vivo B1-maps to find a ‘universal’ solution, similar to universal pTx pulses used in the brain1. Future works will further investigate in-vivo results, and the reliability of the optimization between anatomies.
Acknowledgements
This work was supported by ARSEP, Institut Marseille Imaging, A*midex and France Life Imaging.References
1. Gras V, Vignaud A, Amadon A, Le Bihan D, Boulant N. Universal pulses: A new concept for calibration-free parallel transmission. Magn Reson Med. 2017;77(2):635-643. doi:10.1002/mrm.26148 SMASH
2. Padormo F, Beqiri A, Hajnal JV, Malik SJ. Parallel transmission for ultrahigh-field imaging. Nmr in Biomedicine. 2016;29(9):1145-1161. doi:10.1002/nbm.3313 SMASH 3. Destruel A, Jin J, Weber E, et al. Integrated Multi-Modal Antenna With Coupled Radiating Structures (I-MARS) for 7T pTx Body MRI. IEEE Transactions on Medical Imaging. 2022;41(1):39-51. doi:10.1109/TMI.2021.3103654 SMASH
4. Bosch D, Bause J, Geldschläger O, Scheffler K. Optimized ultrahigh field parallel transmission workflow using rapid presaturated TurboFLASH transmit field mapping with a three-dimensional centric single-shot readout. Magnetic Resonance in Medicine. 2023;89(1):322-330. doi:https://doi.org/10.1002/mrm.29459 SMASH
5. Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med. 2007;57(1):192-200. doi:10.1002/mrm.21120 SMASH
6. Tse DHY, Poole MS, Magill AW, Felder J, Brenner D, Shah NJ. Encoding methods for B1+ mapping in parallel transmit systems at ultra high field. Journal of Magnetic Resonance. 2014;245:125-132. doi:https://doi.org/10.1016/j.jmr.2014.06.006 SMASH
7. Malik SJ, Larkman DJ, Hajnal JV. Optimal linear combinations of array elements for B1 mapping. Magnetic Resonance in Medicine. 2009;62(4):902-909. doi:https://doi.org/10.1002/mrm.22068 SMASH
8. Massire A, Taso M, Besson P, Guye M, Ranjeva JPP, Callot V. High-resolution multi-parametric quantitative magnetic resonance imaging of the human cervical spinal cord at 7T. NeuroImage. Published online 2016. doi:10.1016/j.neuroimage.2016.08.055 SMASH
9. Amadon A, Boulant N, Cloos MA, et al. B1 mapping of an 8-channel TX-array over a human-head-like volume in less than 2 minutes: the XEP sequence, In Proceedings of the Annual Meeting of ISMRM 2010.
10. Brunner DO, Pruessmann KP. B1(+) interferometry for the calibration of RF transmitter arrays. Magn Reson Med. 2009;61(6):1480-1488. doi:10.1002/mrm.21893 SMASH
11. Moortele PF van de, Snyder CJ, DelaBarre L, Adriany G, Vaughan T, Uğurbil K. Calibration Tools for RF Shim at Very High Field with Multiple Element RF Coils : from Ultra Fast Local Relative Phase to Absolute Magnitude B 1 + Mapping. In: ; 2007.
12. Bosch D, Müller F, Scheffler K. Interferometric, Hybrid, and Weighted B+1 Mapping for Expedited RF Calibration of Parallel Transmit Ultrahigh-Field MRI, In Proceedings of the Annual Meeting of ISMRM 2022.
13. Van Damme L, Mauconduit F, Chambrion T, Boulant N, Gras V. Universal nonselective excitation and refocusing pulses with improved robustness to off-resonance for Magnetic Resonance Imaging at 7 Tesla with parallel transmission. Magnetic Resonance in Medicine. 2021;85(2):678-693. doi:10.1002/mrm.28441 SMASH
14. Eichfelder G, Gebhardt M. Local Specific Absorption Rate Control for Parallel Transmission by Virtual Observation Points. Magnetic Resonance in Medicine. 2011;66(5):1468-1476. doi:10.1002/mrm.22927 SMASH
Figures