4408
Accelerated Volumetric Multi-Channel pTx B1+ Mapping at 7T for the Brain and Heart
James L. Kent1, Ladislav Valkovič2,3, Iulius Dragonu4, Mark Chiew1,5,6, and Aaron T. Hess1
1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Oxford Centre for Clinical Magnetic Resonance Research, John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom, 3Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia, 4Research & Collaborations GB&I, Siemens Healthcare Ltd, Camberley, United Kingdom, 5Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 6Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada
1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Oxford Centre for Clinical Magnetic Resonance Research, John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom, 3Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia, 4Research & Collaborations GB&I, Siemens Healthcare Ltd, Camberley, United Kingdom, 5Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 6Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada
Synopsis
Keywords: Parallel Transmit & Multiband, Parallel Transmit & Multiband
Ultra-high field MRI with parallel transmit offers significant advantages but B1+ maps are required to utilize its potential. Acquiring B1+ maps for each channel is time-consuming and limits the application of ultra-high field in clinical practice. In this abstract we present an approach to acquire full 3D multi-channel B1+ maps in 11 seconds. We do this by employing a time-interleaved acquisition of modes strategy using undersampled relative maps and two fast undersampled Sandwich B1+ maps all reconstructed using TxLR, a calibrationless reconstruction for transmit field maps. We demonstrate this approach with simulations and scans in phantom and in the brain.Introduction
B1+ inhomogeneity is severe across the heart and large across the brain at 7T, limiting the utility of ultrahigh-field cardiac imaging. Parallel transmit (pTx) solves this problem but requires multi-channel B1+ maps which are difficult to acquire in a clinically reasonable timeframe and are compounded by issues of cardiorespiratory motion. An adaptation to the presaturation TurboFLASH method1 of acquiring B1+ maps, termed Sandwich, enables faster absolute maps2 that are less sensitive to T1 and maintain flow-insensitivity. In this work we combine three acquisition strategies, Sandwich, B1TIAMO3, and relative transmit maps and reconstruct the data using transmit low rank reconstruction (TxLR)4 to measure multi-channel B1+ maps. Our is aim to reduce the acquisition duration to a single breath-hold or 16 heartbeats. We evaluate the method using simulations, phantom and in vivo brain data.Methods
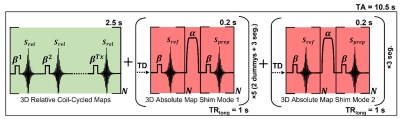
The proposed acquisition scheme is shown in Figure 1. This includes three measurement sections: 1) an undersampled 3D coil-cycled multi-channel relative transmit map, acquired using a spoiled low flip angle gradient echo sequence, one image from each transmit channel is acquired in an interleaved fashion, minimising the influence of magnetisation history. 2) and 3) are 3D undersampled absolute maps with complementary shim modes (CP+ and CP2+).Simulations were performed using retrospectively undersampled in-silico data to establish the achievable acceleration and masking strategy. The in-silico data were generated in Sim4Life (3.4, ZMT) using a model of an 8-channel dipole array around DUKES heart, channel-independent complex Gaussian noise was added to this data. K-space was cropped to 24×24 phase encodes and randomly undersampled using a Poisson disk distribution at rates of 1-16. The central 4×4 region of k-space was maintained.
A 7 Tesla MRI (VB17, Siemens Healthcare) equipped with parallel transmit was used for data acquisition. Data were measured in a water phantom and in vivo brain of a healthy volunteer using an 8Tx/32Rx head coil (Nova Medical).
The 3D relative maps were acquired with TEGRE=1.31ms, TRGRE=3.2ms, bandwidth=490Hz/px, FOV=(250mm)3, matrix=24×24×24, resolution=(10.4mm)3, RF-spoiling, non-selective RF excitation FA=9°, and a total acceleration factor 6 with central 4×4 central region and a different undersampling mask for each transmit channel.
The 3D absolute maps were acquired using the Sandwich2 scheme based on a TurboFLASH sequence, TE=1.26ms, TR=3ms, bandwidth=490Hz/px, FOV=(250mm)3, matrix=24×24×24, resolution=(10.4mm)3, RF-spoiling and total acceleration factor 6 (4×4 central calibration region) with non-selective RF excitation (β). A non-adiabatic hyperbolic secant RF pulse was used as our presaturation (α) with nominal α/β flip angles 90/4.5°, each volume was acquired in 3 segments, each with 32 lines. This was repeated for two acquisition modes, with the first mode preceded by two dummy TRs. The total acquisition time was 8s (2 dummy TRs + 3 segments mode 1 + 3 segments mode 2). Different undersampling masks were used for each shim mode, but the reference and prepared images were acquired using the same mask, with centre-out encoding. The total acquisition time to acquire both the relative and absolute maps was 11 seconds.
The relative, reference and prepared cartesian undersampled k-spaces were concatenated to form a 24×24×24×8×12 (Nkx×Nky×Nkz×NRx×NTx) matrix and jointly reconstructed using the TxLR method4 sliced along the readout direction. The k-space data were Hann filtered, zero-padded to 24×32×32 and fast Fourier transformed back to full image space. The receive channel images were combined using sensitivity maps estimated using ESPIRiT5. Reconstruction was performed offline in MATLAB (R2021a, MathWorks) on an Intel Xeon 28×2.40GHz 128Gb RAM computer. We compare a slice from our 3D maps to a 2D presaturation TurboFLASH method which took 90 seconds to acquire.
Results
Simulation results from synthetic 8Tx/8Rx body images in Figure 2 show acceleration factors up to 6 are achievable with a mean difference to the fully sampled of <1°. Figure 3a shows the 3D dataset acquired in a phantom in 11 seconds. Figure 3b and 3c compares our 3D data to a fully sampled 2D dataset with the difference in part d. Figure 4 shows similar data in the brain of one subject with a mean absolute difference of 0.4°. Reconstruction took 12 minutes.Discussion
Simulations show that acceleration factors of 6 are comfortably achievable and higher are possible. We found that using a small fully sampled region (4×4) aided the reconstruction allowing faster reconstruction times and reduced RMSE, particularly for higher acceleration factors, this also improved our in vivo results. The accelerated data appears spatially smoother than the fully sampled images, indicating further investigation of actual resolution is required. The main limitation currently is the 12 minute reconstruction time for the 3D datasets on our hardware. Although we do not show any in vivo cardiac data, our simulations and brain work suggests that to acquire our accelerated 3D maps in the heart would 8 heartbeats for relative mapping and 8 for absolute mapping, even taking into account the need for segmenting and gating the relative maps. In principle, the two sequences can be acquired together in a single breath-hold or in two subsequent breath-holds. Going forward we aim to apply this method in the heart.Conclusion
We obtain 3D multi-channel absolute B1+ maps in the brain in 11 seconds for an entire transmit array with good accuracy to fully sampled reference maps.Acknowledgements
JK acknowledges the support of EPSRC through an iCASE award in collaboration with Siemens Healthcare Ltd. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z). LV is funded by a Sir Henry Dale Fellowship awarded jointly by the Wellcome Trust and the Royal Society (221805/Z/20/Z) and also acknowledges the support of the Slovak Grant Agencies VEGA (2/0003/20) and APVV (#19–0032). MC is supported by the Canada Research Chairs Program.References
- Chung S, Kim D, Breton E, Axel L. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med 2010;64:439–446.
- Kent, JL, Dragonu, I, Valkovič, L, Hess, AT. Rapid 3D absolute B1+ mapping using a sandwiched train presaturated TurboFLASH sequence at 7T for the brain and heart. Magn Reson Med 2022;1-13.
- Brunheim S, Gratz M, Johst S, et al. Fast and accurate multi‐channel mapping based on the TIAMO technique for 7T UHF body MRI. Magn Reson Med 2018;79:2652–2664.
- Hess AT, Dragonu I, Chiew M. Accelerated calibrationless parallel transmit mapping using joint transmit and receive low‐rank tensor completion. Magn Reson Med 2021;86:2454–2467.
- Uecker M, Lai P, Murphy MJ, et al. ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med 2014;71:990–1001.
Figures
Figure
1: Sequence diagram showing the B1+ time-interleaved
acquisition of modes strategy of acquiring multi-channel relative maps (green)
followed by fast absolute maps using complementary shim modes (red). The absolute
maps excitation (β) and preparation pulse (α) are transmitted using the same
shim mode. Fast absolute maps are acquired using our sandwich scheme and the
use of a 5 ms non-adiabatic hyperbolic secant pulse as our presaturation pulse
allows for improved robustness to B0 inhomogeneity. TA, total acquisition; TD,
time delay.
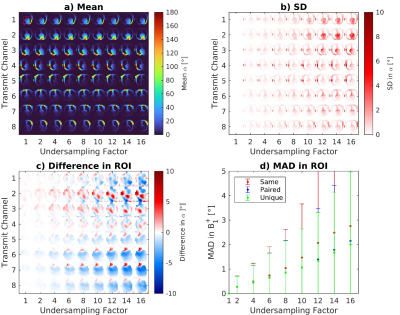
Figure
2: Simulation results from synthetic body images showing the mean a) and
SD b) for multi-channel parallel transmit B1+ maps
across various undersampling factors 1-16. 50 repeats with different masks were performed. Subfigure c) shows the mean absolute difference (MAD) across these repeats to the fully sampled k-space in a region of interest (red box in subfigure a)) summarised in d) for the three masking strategies
tested. These were: ‘same’ identical masks for
all, ‘paired’ same mask for reference/prepared images and ‘unique’ all transmit modes used a different mask.
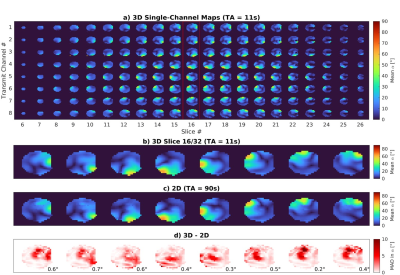
Figure
3: 3D multi-channel maps acquired in a water phantom in 11 seconds with the proposed method are
shown in a) a single slice from this dataset (outlined in red) is shown
in b). 2D multi-channel maps are shown in c) which were acquired
fully sampled using the presaturation TurboFLASH method with the difference to b)
shown in d) along with the average MAD of each transmit channel in the
bottom right. TA, total acquisition; MAD, mean absolute difference.
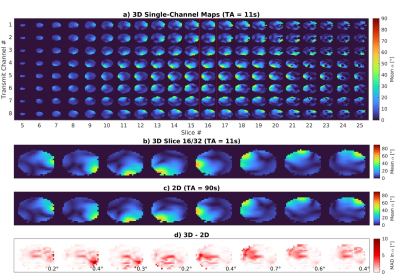
Figure
4: 3D multichannel maps acquired in the brain in 11 seconds with the proposed method are
shown in a) a single transverse slice from this dataset (outlined in
red) is shown in b). 2D multi-channel maps are shown in c) which
were acquired fully sampled using the presaturation TurboFLASH method with the
difference to b) shown in d) along with the average MAD of each
transmit channel in the bottom right. TA, total acquisition; MAD, mean absolute
difference.
DOI: https://doi.org/10.58530/2023/4408