4404
Static and dynamic parallel transmission (pTx) for human cardiac MRI at 21.0 T1Max-Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 3Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max-Delbrück Center for Molecular Medicine, Berlin, Germany, 4MRI.TOOLS, Berlin, Germany
Synopsis
Keywords: Shims, RF Arrays & Systems, Cardiovascular
Transmission field inhomogeneities at ultrahigh and extreme field MRI can be offset using static or dynamic pTx. Responding to the challenges and recognizing the opportunities of cardiac MRI, this abstract examines the feasibility of parallel transmission (pTx) using self-grounded bow-tie (SGBT) RF array configurations for static and dynamic B1+ homogenization of the heart at 21.0T. Our results demonstrate that static pTx provides limited performance at 21.0 T, but dynamic pTx enables uniform heart excitation at 21.0TIntroduction
The benefit of ultrahigh field (UHF, B0≥7.0T) MR is challenged by transmission field (B1+) inhomogeneities due to RF wavelength shortening and destructive/constructive electromagnetic field (EMF) interferences. To mitigate spatial B1+ variation, local RF transceiver arrays [1] were established to support B1+ shimming through an adaptation of multichannel transmission [2]. Pushing the boundaries and unlocking the potential of extreme field MR (EF, B0≥14.0T) requires unraveling and leveraging the electrodynamics of the short wavelength regime. Responding to the challenges and recognizing the opportunities of CMR at 21.0T, this abstract elucidates the electrodynamic constraints at high spin excitation frequencies, explores the benefits of multi-transmission using a self-grounded bow-tie antenna building block (SGBT) RF array, and demonstrates the feasibility of static and dynamic parallel transmission (pTx) using optimized kT points at 21.0TMethods
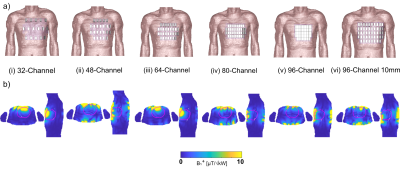
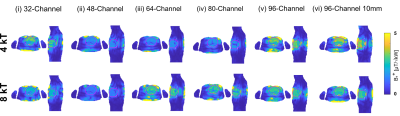
For the design of RF arrays, a self-grounded bow-tie antenna building block (SGBT) [3] was implemented in numerical EMF simulations. The geometric antenna dimensions were scaled to the 1H resonance frequency at 21.0T (f=900MHz). A baseline setup with a 32-channel SGBT RF array was implemented at 21.0T and placed on the upper torso of a human voxel model. Next, we increased the channel count to 48, 64, 80, and 96 SGBT Tx elements. These setups provide the highest possible antenna density covering the same upper torso area as the baseline setup. Additional setups were established to optimize the decoupling of the high-density arrays at 21.0T by increasing the nearest neighbor distance to 10mm. EMF simulations were performed in CST using the human voxel model Duke [4]. B1+ and maximum local specific absorption rate over 10 g tissue (SAR10g) were examined for each RF array at 21.0T. A static and dynamic pTx approach [2] was employed to enhance the B1+ efficiency and homogeneity for a region of interest (ROI) covering the heart. Dynamic pTx was performed with 4 and 8 tailored kT-points [5], a series of RF sub-pulses, and gradient blips with the goal of 3D flip angle (FA) homogenization (CoV(FA)) targeting the whole heart [6]. The pulse design was done in Matlab 2019b using the small-tip-angle approximation (STA) for a nominal target FA of 10° across the whole heart [6-8]. The total pulse duration of the kT point pTx pulses was scaled to 1ms for an inserted power (PIn) of 1kW to compare dynamic and static pTx approaches. The obtained FA maps (FA= γ B1+ τ) were scaled into B1+ efficiency maps where the forward power (Pfwd) of the kT points was scaled to 1ms.Results
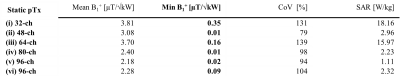
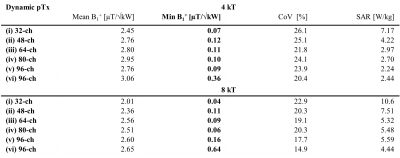
For the baseline setup phase and amplitude optimized static pTx showed (Figure 1b) a maximized minimum B1+ROI < 0.35 µT/√kW, which comes with a high maximum local SAR10g of 18.16W/kg and a CoV of 131% (Table 1). CoV across the heart was reduced to 26.1% with dynamic pTx with tailored 4 kT-point pulses (Figure 2). This approach provided minimum B1+ROI = 0.07 µT/√kW with maximum local SAR10g =7.17 W/kg (Table 2). Increasing the channel count to 96 Tx elements facilitated a CoV of 23.9% with minimum B1+ROI=0.09 µT/√kW and maximum local SAR10g=2.24 W/kg. The nearest neighbor decoupling optimized 96-channel setup (vi) enabled CoV=20.4% with minimum B1+ROI=0.36 µT/√kW and maximum local SAR10g=2.44 W/kg for dynamic pTx with tailored 4 kT-point pulses. Using 8 kT points for the same array configuration setup facilitated CoV=14.9%, minimum B1+ROI=0.64 µT/√kW, and maximum local SAR10g=4.44 W/kg.Discussion and Conclusion
Our EMF simulations demonstrate the B1+-uniformity and efficiency challenges of CMRI at 21.0T. At this field strength, static pTx showed limited performance. Dynamic pTx using tailored 4 kT-point pulses revealed a more uniform FA distribution than static pTx (RF shimming). The improved CoV was associated with increased SAR levels and reduced B1+ efficiency. We increased the number of kt-points from 4 to 8 facilitated CoV enhancement. However, SAR was also raised, which presents a practical concern. Increasing the Tx-channel count offsets this practical obstacle with lower CoV and SAR. Dynamic pTx with 8 kT-points combined with the decoupling optimized 96-channel setup showed the best performance. CoV was improved versus static pTx while a minimum B1+ROI = 0.64 µT/√kW and a maximum local SAR10g < 4.44 W/kg were achieved. An enhanced Tx channel count with optimized decoupling as well as more kT points will be beneficial to improve CoV across the heart further.To conclude, static pTx shimming provides limited performance at 21.0T. On the other hand, dynamic pTx enables more uniform heart excitation at 21.0T. This finding is heartening and provides the technical foundation for explorations into cardiac MRI at 21.0T.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program under grant agreement No 743077 (ThermalMR).References
1. Zhu Y (2004) Parallel Excitation with an Array of Transmit Coils. 51:775-784.
2. Padormo F, Beqiri A, Hajnal JV, Malik SJ (2016) Parallel transmission for ultrahigh-field imaging. NMR in Biomedicine 29:1145-1161.
3. Eigentler TW, Kuehne A, Boehmert L, Dietrich S, Els A, Waiczies H, Niendorf T (2021) 32-Channel self-grounded bow-tie transceiver array for cardiac MR at 7.0T. Magn Reson Med 86 (5):2862-2879.
4. Christ A, Kainz W, Hahn EG, Honegger K, Zefferer M, Neufeld E, Rascher W, Janka R, Bautz W, Chen J, Kiefer B, Schmitt P, Hollenbach HP, Shen J, Oberle M, Szczerba D, Kam A, Guag JW, Kuster N (2010) The Virtual Family - Development of surface-based anatomical models of two adults and two children for dosimetric simulations. Physics in Medicine and Biology 55.
5. Cloos MA, Boulant N, Luong M, Ferrand G, Giacomini E, Le Bihan D, Amadon A (2012) KT-points: Short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magnetic Resonance in Medicine 67:72-80.
6. Aigner CS, Dietrich S, Schmitter S (2021) Three-dimensional static and dynamic parallel transmission of the human heart at 7 T. NMR in Biomedicine 34:1-15.
7. Grissom WA, Khalighi M-M, Sacolick LI, Rutt BK, Vogel MW (2012) Small-tip-angle spokes pulse design using interleaved greedy and local optimization methods. Magn Reson Med 68:1553-1562.
8. Cao Z, Yan X, Grissom WA (2016) Array-compressed parallel transmit pulse design. Magn Reson Med 76:1158-1169.
Figures