4400
Development of Small Animal PET-insert Compatible RF Coils for Simultaneous 129Xe MRI/15O2-PET Brain Perfusion Measurements1Physics and Astronomy, Western University, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3Siemens Healthcare Limited, London, ON, Canada, 42Lawson Health Research Institute, London, ON, Canada
Synopsis
Keywords: RF Arrays & Systems, Hyperpolarized MR (Gas)
This study aims to present two birdcage radiofrequency (RF) coils for the use of simultaneous hyperpolarized Xenon-129 (129Xe) MRI and 15O2 PET imaging for brain perfusion measurements. Coils were designed for use with a 2.89T Siemens scanner, or 34.05MHz, one using a low pass filter design, and the other a high pass filter design.Introduction
The use of laser-polarized Xenon-1291 (129Xe) as a novel contrast agent for magnetic resonance imaging (MRI) has been shown to be effective for functional2 and structural imaging of the brain3 and other organs4, 5. The original interest of one of the co-inventors was to use 129Xe to better understand the brain, namely directly imaging the effect of anesthesia on brain function and cognition6. As a result of over two decades of research and development within the field, both brain2, 3 and lung imaging7, 8 have been made possible in animals and humans, reliable polarizers are now commercially available9. 129Xe-based lung imaging for imaging disease progression is clinically accepted in the United Kingdom10, and FDA approval in the United States is expected this year11. Our research program focuses on the development of advanced novel imaging techniques for assessing brains and consequently the development of hardware needed to conduct those studies. 129Xe-based imaging could transform our methods of mapping grey and white matter12-14, perfusion3, and targeted drug and cell tracking techniques15 by improving sensitivity over other MRI methods such as conventional proton MR imaging, and reaching beyond the resolution limitations of PET. Birdcage coils have been widely used in MR imaging as their RF homogeneity and SNR exceed that of common linear coils16. High-pass birdcage coils are constructed by adding two capacitors of equal value on either end of the equivalent circuit, these capacitors sit perpendicular to the main body of the coil. Low-pass birdcage coils have one capacitor in the middle of the rung, aligned parallel with the main body of the coil17. In this proof-of-concept study, we propose to build two same-size 129Xe rat-head-sized RF coils for conducting simultaneous [15O] PET and hyperpolarized 129Xe MRI brain perfusion imaging. Two main birdcage design RF coils (low- and high-pass) were built to determine the optimal coil design for the multi-modalities brain perfusion measurements inside a NuPET small animal PET insert (Cubresa Inc, Winnipeg Manitoba).Methods
Both high-pass and low-pass coils were tuned to 34.05MHz at 2.89T inside the PET insert, with 8 rungs on each coil. The coil was built to 75 mm in length, with a diameter of 45mm. Coils were matched to approximately 50Ω. Rungs were constructed with copper tape, and each loop element was tuned with non-magnetic capacitors (Passive Plus, USA) and inductors (CoilCraft, USA). The dominant resonant frequency mode was chosen due to its low impedance17. Hyperpolarized 129Xe gas was obtained from a turn-key, spin-exchange polarizer system (Polarean 9800 129Xe polarizer). The initial 129Xe polarization was 15%. 129Xe spectrum and images were acquired in a 3T PET/MRI (Siemens Biograph mMR, Siemens Healthineers, Erlangen, Germany) scanner using whole-body gradients. A Fast Gradient Recalled Echo sequence was utilized using the following parameters: Matrix Size=64x64; Slice thickness=250mm; TE/TR=2.04/20ms; BW=660Hz/pixel; Flip angle=11o (a Constant Flip Angle approach was used); and FOV=100x100mm2.Results
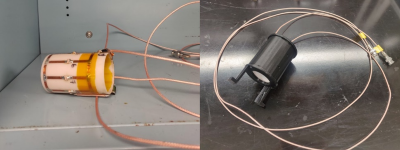
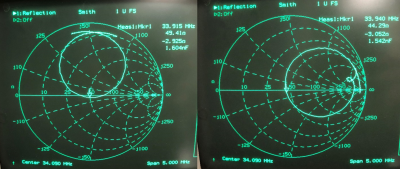
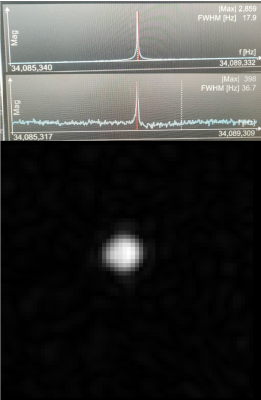
The low-pass and high-pass RF coils are shown in Fig.1. Coils were measured using a vector network analyzer. The low-pass circuit has a sensitivity of -19.8 dB for channel 1 and -27.6 dB for channel 2 (Fig.2, right). The low-pass-coil is matched to 44.3Ω with a 2.3 Ω phase (Fig.3, right). Input power was calculated to be 7V. The high-pass-circuit had a sensitivity of -22.7 dB for channel 1 and -29.3 dB for channel 2 (Fig.2, left). The impedance of the coil was 49.4Ω with a 3.0Ω phase (Fig.3, left). Input power was calculated to be 5V. Fig.4 shows the 129Xe spectra obtained for both coils and the 2D 129Xe phantom image obtained with the high-pass RF coil.Discussion and Conclusions
We were able to build two rat-head-sized 129Xe RF coils fitting into NuPET small animal PET insert resonating at the 129Xe resonance frequency at the 3T MRI system. Initial phantom scans indicated that both coils have demonstrated good sensitivity, and therefore they can be used in the simultaneous in-vivo [15O] PET and hyperpolarized 129Xe MRI brain perfusion imaging measurement. Further in-vivo PET/MRI scans should help us to decide which circuit design provides better SNR on the 129Xe side and causes minimal PET attenuation. While both coils provided clear 129Xe images, the high-pass birdcage RF coil circuit design places capacitors at the end of the rungs, allowing for axial and radial symmetry and likely less potential for PET signal attenuation coming from the central part of the high-pass coil where the animal head is normally placed during the PET/MRI scan. On the other hand, low-pass coils are only radially symmetric, as the capacitors are at the middle of the rungs, and therefore the PET transparency of the central part of the coil is affected. High-pass coils set the capacitors and other main coil components outside of the FOV of the PET insert, and therefore are better suited for use in an MR/PET setting. This work will be expanded upon by imaging with and without the PET insert.Acknowledgements
We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada, R5942A04.References
1. Albert MS, Cates GD, Driehuys B, et al. Biological magnetic resonance imaging using laser-polarized 129Xe. 10.1038/370199a0. Nature. 07/21/print 1994;370(6486):199-201.
2. Mazzanti ML, Walvick RP, Zhou X, et al. Distribution of hyperpolarized xenon in the brain following sensory stimulation: preliminary MRI findings. PLoS One. 2011;6(7):e21607. doi:10.1371/journal.pone.0021607
3. Rao MR, Stewart NJ, Griffiths PD, Norquay G, Wild JM. Imaging Human Brain Perfusion with Inhaled Hyperpolarized (129)Xe MR Imaging. Radiology. Feb 2018;286(2):659-665. doi:10.1148/radiol.2017162881
4. Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, et al. Hyperpolarized (13)C MRI: Path to Clinical Translation in Oncology. Neoplasia. Jan 2019;21(1):1-16. doi:10.1016/j.neo.2018.09.006
5. Hamedani H, Kadlecek S, Ruppert K, et al. A Technique for Quantitatively Measuring Gas Uptake in the Lung and its Distribution to the Kidneys Using Hyperpolarized Xenon-129 MR Imaging. 2018:1082.
6. Fox MS, Couch MJ, Albert MS. Chapter 22 Magnetic Resonance Imaging of the Brain using Hyperpolarized 129Xe. Hyperpolarized Xenon-129 Magnetic Resonance: Concepts, Production, Techniques and Applications. The Royal Society of Chemistry; 2015:407-425.
7. Mugler JP, 3rd, Altes TA. Hyperpolarized 129Xe MRI of the human lung. J Magn Reson Imaging. Feb 2013;37(2):313-31. doi:10.1002/jmri.23844
8. Cleveland ZI, Cofer GP, Metz G, et al. Hyperpolarized Xe MR imaging of alveolar gas uptake in humans. PLoS One. Aug 16 2010;5(8):e12192. doi:10.1371/journal.pone.0012192
9. Kaushik SS, Cleveland ZI, Cofer GP, et al. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med. Apr 2011;65(4):1154-65. doi:10.1002/mrm.22697
10. Wild JM, Collier G, Marshall H, et al. P283 Hyperpolarised Gas MRI – a pathway to Clinical Diagnostic Imaging. Thorax. 2015;70(Suppl 3):A220.3-A221. doi:10.1136/thoraxjnl-2015-207770.419
11. Polarean Imaging moving forward with Phase III trial of hyperpolarised 129-Xenon. https://olvdukeedu/news/polarean-imaging-moving-forward-with-phase-iii-trial-of-hyperpolarised-129-xenon/.
12. Mugler JP, Driehuys B, Brookeman JR, et al. MR imaging and spectroscopy using hyperpolarized129Xe gas: Preliminary human results. Magnetic Resonance in Medicine. 1997;37(6):809-815. doi:10.1002/mrm.1910370602
13. Kershaw J, Nakamura K, Kondoh Y, Wakai A, Suzuki N, Kanno I. Confirming the existence of five peaks in 129Xe rat head spectra. Magn Reson Med. Apr 2007;57(4):791-7. doi:10.1002/mrm.21186
14. Wakai A, Nakamura K, Kershaw J, Kanno I. In vivo MR spectroscopy of hyperpolarized Xe-129 in rat brain. International Congress Series. 2004;1265:139-143. doi:10.1016/j.ics.2004.04.063
15. Zhang B, Guo Q, Luo Q, et al. An intracellular Diamine Oxidase Triggered Hyperpolarized 129Xe Magnetic Resonance Biosensor. 10.1039/C8CC07822J. Chemical Communications. 2018;doi:10.1039/C8CC07822J 16. Ahmad, S. F., Kim, Y. C., Choi, I. C., & Kim, H. D. (2020). Recent Progress in Birdcage RF Coil Technology for MRI System. Diagnostics (Basel, Switzerland), 10(12), 10
17. https://doi.org/10.3390/diagnostics10121017 17. Kim, Y. C., Kim, H. D., Yun, B.-J., & Ahmad, S. F. (2020). A Simple Analytical Solution for the Designing of the Birdcage RF Coil Used in NMR Imaging Applications. Applied Sciences, 10(7), 2242. MDPI AG. Retrieved from http://dx.doi.org/10.3390/app10072242
Figures