4393
Self-activatable photodynamic-immunotherapy based on poly(thioketal) conjugated with photosensitizers1Guangzhou First People’s Hospital, Guangzhou, China
Synopsis
Keywords: Multimodal, Molecular Imaging, MRI Near Infrared Ray
Photodynamic therapy is a non-invasive and controllable modality with potential as a novel cancer treatment strategy. However, the ROS production efficiency of PDT is restricted to hydrophobic characteristics and aggregation caused quenching of PSs. Herein, we designed a ROS self-activatable polymer (PTKPa) to suppress photosensitizers ACQ and elevate ROS production capacity. This platform shows therapeutic outcomes in both cells and orthotopic mouse model, which can significantly elevate the intracellular ROS level upon 660nm laser irradiation, induce immunogenic cell death (ICD) to activate antitumor immunity. This work provides a general strategy enhancing tumor photodynamic therapeutic effects.Introduction
Cancer remains a global health concern. Although many therapeutic methods have been developed for cancer treatment, such as surgery, radiotherapy, chemotherapy, and immunotherapy [1, 2], the clinical therapeutic efficacies are still unsatisfactory. Notably, Photodynamic therapy (PDT) has emerged as a new cancer therapy strategy in recent years [3]. Compared with chemotherapy or radiotherapy, PDT is non-invasive, spatiotemporally controllable, and presents no drug-resistant modality [4]. PDT was a clinically approved minimally invasive therapeutic procedure when Photosensitizers (PSs) irradiated by an appropriate wavelength light source to convert oxygen to reactive oxygen species (ROS), especially singlet oxygen (1O2) can exert a selective cytotoxic activity causing malignant cells irreversible damage[5,6].However, the efficiency of ROS production was restricted to the hydrophobic characteristics and aggregation-caused quenching (ACQ) of photosensitizers[7].It is urgent to develop novel strategies to overcome these shortages of PSs. Herein, we developed a ROS self-activatable nano system PTKPa NPs for controllable release of photosensitizers, amplifying the effect of PDT, and enhancing antitumor immune responses.Method
As scheme1 dipicted,the photosensitizer pheophorbide A (Ppa) can be conjugated with polyethylene glycol by 1O2-activatable poly(thioketal) bond (PTK-SS) to form the amphiphilic PEG-TK-Pa polymer, which can self-assemble to form PTKPa NPs.We design PSDPa as a contol. The experiments were carried on the synthesized PTKPa and PSDPa to characterize their stability, diameter, ROS-activation and generation capacity, magnetic resonance imaging capability,Ppa cumulative release Then,we employmed a serious experiments on 4T1 cells in vitro, such as MTT,detection of apoptosis, ICD effect (CRT, HMGB1,ATP). And we also validate biodistribution,MRI, analysis tumor growth inhibition on 4T1 tumor-bearing mice model.Result And Discussion
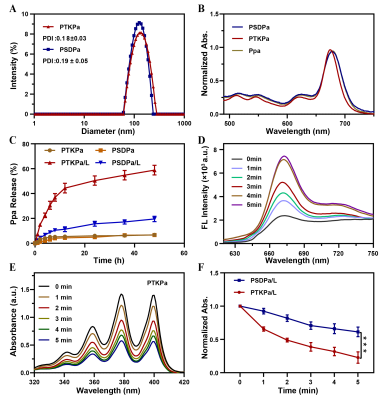
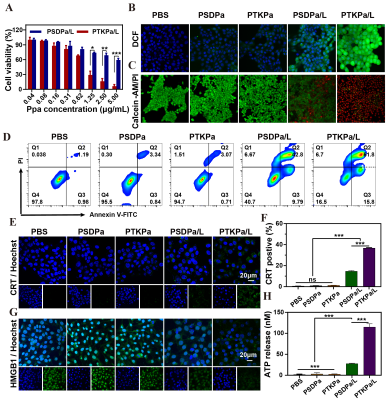
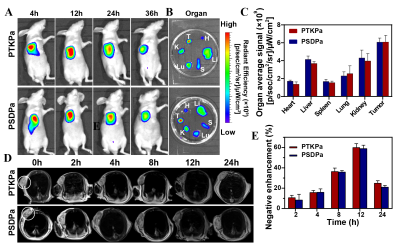
PTKPa NPs(131.9 nm) and PSDPa NPs (137.1 nm )had uniform size distributions, satisfactory dispersibility and stability. PTKPa NPs and PSDPa NPs exhibited similar absorbance to Ppa at 677nm that means Ppa was successfully loaded. Hence, we presumed that, upon 660nm irradiation, ROS causes degradation of PTKPa NPs and Ppa release, generating abundant ROS to achieve a ROS self-amplified cycle.The Ppa cumulative release amount of 58.8 % when PTKPa NPs were exposed to 660 nm , while PSDPa was 19.6%.We employ the 677nm fluorescence intensity variation to confirm the binding process between albumin and Ppa,which suggested PTKpa can overcome ACQ effect and improve Ppa's bioavailability. The UV/Vis absorbance of ABDA indicates that PTKPa NPs possess higher singlet oxygen generation capacity than PSDPa NPs. We deduced from the above-mentioned results that a PTKPa NPs can be activated by ROS, accelerate Ppa release and elevate ROS levels to amplify PDT efficacy in the physiological environment.The PDT efficiency of PTKPa NPs or PSDPa NPs was further detected with 1O2 detector 2′,7′-dichlorofluorescin diacetate, under 660nm laser irradiation, 4T1 cells incubated with PTKPa NPs exhibited the strongest and most uniform green fluorescence.Hence, we have verified the capability of PTKPa NPs to elevate intracellular ROS level for causing amplified oxidative stress upon 660nm laser irradiation. The MTT results of PTKPa NPs and 660nm laser treated 4T1 cells' viability dropped from 100% to 13%. While PSDPa NPs exerted low cell-killing activity (53%), which signified that PTKPa NPs had higher Ppa-triggered oxidative stress capacity than PSDPa NPs in vitro. The CLSM and FCM demonstrated, upon laser irradiation, the majority of 4T1 cells pre-treated PTKPa NPs were labeled as dead cells with a total apoptosis ratio of 77.6%, higher than PSDPa NPs (51.6%) groups.
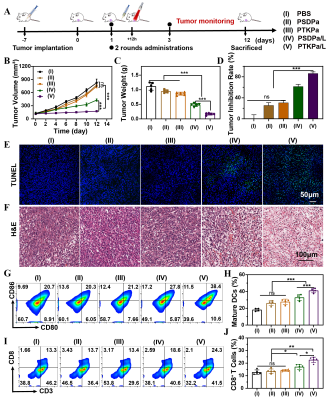
The group of PTKPa NPs combined with 660nm laser had the smallest incremental tumor volume compared to the initial volume, with a tumor growth inhibition rate of 86.6 ± 2.8%, and that of PSDPa NPs + 660 nm laser was 61.6 ± 4.1%. In addition, the most severe morphology variation and tissue necrosis of tumors were observed in the PTKPa NPs combined 660 nm laser, PSDPa NPs combined 660 nm. Similar results were obtained after TUNEL staining, the green fluorescence of CLSM pictures as evidence We then measured the immune cells in 4T1 tumor bearing mice. The recent studies have shown that PDT can induce ICD by ROS-caused damage at the endoplasmic reticulum membrane, which can recruit dendritic cells (DCs) to tumor tissues and boost dendritic-cell migration, antigen uptake and maturation in the tumor-draining lymph nodes [8-11]. Additionally, mature DCs were able to antigen presentation to T cells, facilitated cytotoxic T lymphocytes (CTLs) (CD8+ T cells) activation and responsible for exerting cytocidal effects[3, 12, 13]. Upon laser irradiation, the mice injected with PTKPa NPs, the frequency of DCs maturation up to 39.2 ± 2.8%, almost 2.0-fold higher than that of the PBS group.And the frequency of CD8+ T cells in PTKPa NPs increased from 11.5 ± 1.0% to 24.3 ± 0.6% compared to that of the PBS group. In short, results of the current study illustrate that PTKPa NPs can amplified oxidative stress and facilitate ICD for improving photodynamic-immunotherapy efficiency.
Conclusion
In summary, we have successfully designed a ROS self-activatable polymer PTKPa, capable of enhancing photodynamic therapeutic effect and regulating the maturation of DCs to elicit a robust immune response.The results of in vivo experiment illustrated that PTKPa induced PDT can suppress the tumor growth evidently and activate immune systems dramatically with an increase in CD8+T cells, mature DCs.In conclusion, this work offers a promising strategy for improving photodynamic-immunotherapy efficacy in cancer therapy.Acknowledgements
No acknowledgement found.References
[1]. Miller, K.D., et al., Cancer treatment and survivorship statistics, 2019. CA: a cancer journal for clinicians, 2019. 69(5) 363-385.
[2]. Kennedy, L.B. and A. Salama, A review of cancer immunotherapy toxicity. CA Cancer J Clin, 2020. 70(2) 86-104.
[3]. Agostinis, P., et al., Photodynamic therapy of cancer: An update. CA: A Cancer Journal for Clinicians, 2011. 61(4) 250-281.
[4]. Xie, J., et al., Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem Soc Rev, 2021. 50(16) 9152-9201.
[5] Dai-Fukumura-And-Rakesh Dennis E. J. G. J. Dolmans. Photodynamic therapy for cancer[J]. Nat Rev Cancer, 2003, 3(5 ): 380-387.
[6] Patrizia Agostinis, Berg Kristian, Cengel Keith-A, et al. Photodynamic therapy of cancer: An update[J]. CA: A Cancer Journal for Clinicians, 2011, 61(4): 250-281.
[7] Deng K, Yu H, Li J, Li K, Zhao H, Ke M, Huang S, Dual-step irradiation strategy to sequentially destroy singlet oxygen-responsive polymeric micelles and boost photodynamic cancer therapy. Biomaterials 275(2021)120959.
[8]. Castano, A.P., P. Mroz and M.R. Hamblin, Photodynamic therapy and anti-tumour immunity. Nature Reviews Cancer, 2006. 6(7) 535-545.
[9]. Tanaka, M., et al., Immunogenic cell death due to a new photodynamic therapy (PDT) with glycoconjugated chlorin (G-chlorin). Oncotarget, 2016. 7(30) 47242-47251.
[10]. Korbelik, M., Induction of tumor immunity by photodynamic therapy. J Clin Laser Med Surg, 1996. 14(5) 329-34.
[11]. Obeid, M., et al., Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Medicine, 2007. 13(1) 54-61.
[12] Castano, A.P., P. Mroz and M.R. Hamblin, Photodynamic therapy and anti-tumour immunity. Nature Reviews Cancer, 2006. 6(7)535-545.
[13] Li, X., et al., Clinical development and potential of photothermal and photodynamic therapies for cancer. Nature reviews. Clinical oncology, 2020. 17(11) 657-674.
Figures
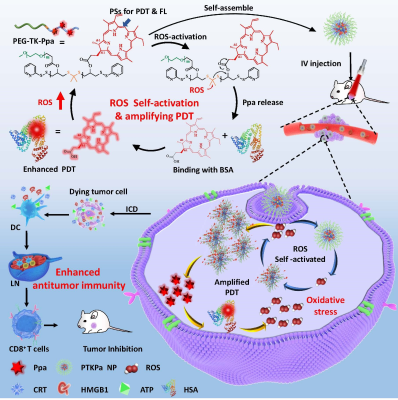
Scheme 1. Self-activatable photodynamic-immunotherapy based on poly(thioketal) conjugated with photosensitizers. (A) Chemical structure of ROS self-activatable nano system PEG-TK-Pa consisting of ROS-responsive TK group, ROS-generating agent Ppa, hydrophilic PEG. Upon 660nm laser irradiation, the ROS produced by conjugated Ppa as a triggering agent initiates poly(thioketal) cleavage with the release of Ppa, which in turn produces massive ROS, causing severe oxidative stress. (B) Schematic illustration the process for enhancing cancer photodynamic-immunotherapy.