4392
Electric Dipole Based Metasurfaces Enhance Targeted RF Power Deposition of Thermal Magnetic Resonance at 7.0T, 9.4T and 10.5T1Berlin Ultrahigh Field Facility (B.U.F.F.), Max-Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, Berlin, Germany, 2The Charité – Universitätsmedizin, Berlin, Germany, 3Department of Neurobiology, Weizmann Institute of Science, Rehovot, Israel, 4Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max-Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
Keywords: Interventional Devices, RF Arrays & Systems, Metamaterial surface
This work proposes the feasibility of passive metasurface (MS) to enhance the RF power deposition for Thermal Magnetic Resonance based treatment of brain tumors. An 8 channel hybrid RF applicator combining dipole antenna and passive MS is designed and evaluated at 7.0 T (300 MHz), 9.4 T (400 MHz) and 10.5 T (450 MHz). MS with metal strips inside waterbolus was designed which generates expected resonant modes of the thermal intervention frequency. The result demonstrated use of passive metamaterial surface in RF applicator enhances the RF power deposition inside target volume for targeted RF heating of deep seated brain tumor.PURPOSE
Metamaterial planar surfaces metasurface (MS) are of proven value to enhance the performance of RF antenna tailored for MRI due to the extra degrees of freedom for shaping electromagnetic fields1-5. MS can be used for SAR10g reduction, for B1+ and SNR enhancement and for RF E-field focusing. Thermal Magnetic Resonance (Thermal MR) is a hyperthermia variant that adds a therapeutic dimension to diagnostic MRI. Thermal MR integrates RF induced heating, MR temperature mapping, anatomical and functional MRI in a single, multipurpose RF applicator permitting supervised targeted temperature modulation6. Clinical benefits have been reported for localized thermal therapy as potent sensitizer of chemo and radiotherapy for various cancers and for targeted drug release6-9. Recognizing this opportunity this work examines the applicability of passive MS to enhance the efficacy of targeted RF power deposition en route to Thermal MR based treatment of brain tumors. For this purpose, an 8 channel hybrid RF applicator combining dipole antenna and passive MS is designed and evaluated at 7.0 T (300 MHz), 9.4 T (400 MHz) and 10.5 T (450 MHz) in clinically realistic brain tumor models.METHODS
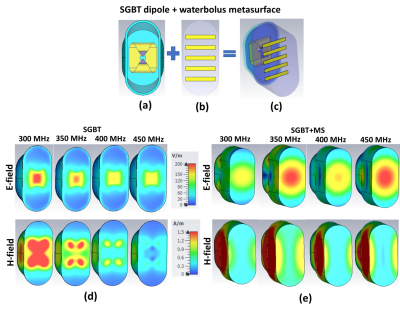
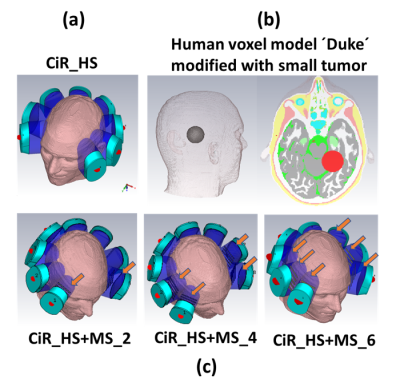
The RF applicator combines eight compact SGBT10 dipole antenna (size: 42.3x46.3x2.5 mm3) (Figure 1a) to support MRI at 300MHz (B0 = 7.0 T), 400MHz (B0 = 9.4 T) and 450MHz (B0 = 10.5T). Each SGBT dipole uses a water bolus placed between the radiating element and the surface of the object under investigation to enhance the efficiency and directivity for targeted RF heating. A circular RF applicator array (CiR_HS) was designed using a horse-shoe shape (arc=2700) coverage of the human head (Figure 2a). For RF targeted heating, each waterbolus was modified by adding a set of 5 conducting copper strips (size (45x6x4 mm) with strip gap 10mm (Figure 1b,1c). This waterbolus (εr =78.4) serves as a dielectric substrate for added copper strips, which supports a set of electromagnetic modes similar to metasurface. Therefore, the modified waterbolus behave as a metasurface (MS). The MS has (similar as TM11 mode13) strong axial electric-field mode with maximum in the center and parallel to the direction of propagation at 350 and 450 MHz (Figure 1e). Three RF applicators were designed (Figure 2c) with 2, 4 or 6 number of MS with the goal to increase SAR10g inside the target volume (TV) of the brain tumor and to spare remote healthy tissue.For RF heating multiple discrete frequencies f= 300, 350, 400, 450MHz were used. EMF simulations (CST Studio Suite 2020) were performed using the human voxel model ‘Duke' (IT'IS Foundation Zürich, CH)6 with an intracranial sphere (r= 2cm radius) modification that represents a small tumor6 (volume=33.5ml, σtumor = 1.15 S/m, εrtumor = 66.5) in the right parietal region of the brain to mimic a clinical scenario (Figure 2b). Postprocessing was conducted (MATLAB 2020) to calculate B1+ field, SAR10g and targeted RF heating optimization. A time and frequency multiplexed algorithm was used11 to provide globally optimal excitation vectors defining the phase and amplitude setting for each RF channel. The resulting SAR distribution of the interfering incident E-fields are tailored for heating the target volume with the goal to reduce RF exposure to healthy tissues. The focusing abilities of the proposed RF applicators were evaluated using the metrics SAR10g and tumor coverage TCx11-12. The tumor coverage TC25, TC50, TC80 and TC100 detail the fraction of the tumor volume enclosed in the 25%,50%, 80% and 100% isolines of peak SAR10g11-12.RESULTS
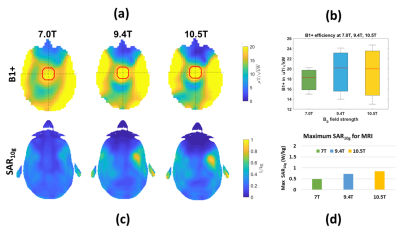
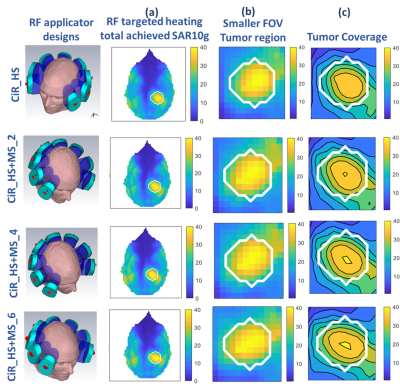
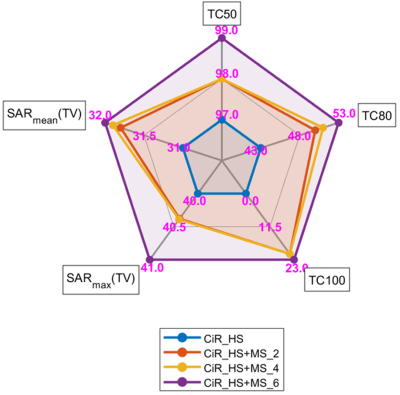
Our EMF simulations demonstrate that the CiR_HS RF applicator is very well capable to create a B1+ transmission fields suitable for MRI at 7T, 9.4T, 10.5T (Figure 3a). Mean and maximum value of B1+ obtained for a ROI placed in the center of the brain (red circle) were increased at higher frequencies at cost of B1+ uniformity (Figure 3b).Figure 4a summarizes the results obtained for targeted RF heating using the excitation vector optimization algorithm at frequencies of 300, 350, 400, 450 MHz. The use of MS facilitates improvement of mean SAR10g (32W/kg) in tumor TV and adding 6 MS to the RF applicator (CiR_HS+MS_6 design) increases maximum SAR10g to 41 W/kg (Figure 4a, 4b) compared to CiR_HS.Significant enhancement of TC80 and TC100 was obtained for RF applicator with MS compare to without MS of SGBT dipole RF applicator CiR_HS. The TC80 of RF applicators CiR_HS+MS_2, CiR_HS+MS_4 , CiR_HS+MS_6 are 50%, 51%, 52% respectively, which are 16%, 19%, 21% increase over CiR_HS design with TC80=43% (Figure 4c, 5). CiR_HS does not provide any TC100. Adding metamaterial enables TC100 of 20% (CiR_HS+MS_2), 21% (CiR_HS+MS_4) and 23% (CiR_HS+MS_6) (Figure 4c, 5).
DISCUSSIONS & CONCLUSIONS
This study demonstrates the feasibility of a circular horse-shoe 8-channel hybrid RF applicator which combines SGBT antenna building blocks with passive metasurfaces for MRI and for targeted RF induced heating of deep-seated brain tumors at 7.0T, 9.4T, 10.5T. Our findings show that modifying water boluses as passive metasurfaces facilitates improved SAR focusing inside the tumor TV and enhances tumor coverage. Our approach of using a water bolus makes the use of extra bulky and expensive dielectric substrates for the MS obsolete. Our simulations provide the technical foundation for the implementation and application of the proposed RF applicator design.Acknowledgements
This project was funded in part by an advanced ERC grant (EU project Thermal MR: 743077).This project is a join collaboration between Max Delbrück Center for Molecular Medicine (MDC) and the Weizmann Institute of Science, Israel as apart of Helmholtz International Research School (HIRS) for Imaging and Data Science from the NAno to the MESo (iNAMES).References
1. Schmidt R, Webb A. Metamaterial Combining Electric- and Magnetic-Dipole-Based Configurations for Unique Dual-Band Signal Enhancement in Ultrahigh-Field Magnetic Resonance Imaging. ACS Applied Materials & Interfaces 2017 9 (40), 34618-34624. DOI: 10.1021/acsami.7b06949
2. Webb A, Shchelokova A, Slobozhanyuk A, Zivkovic I, Schmidt R. Novel materials in magnetic resonance imaging: high permittivity ceramics, metamaterials, metasurfaces and artificial dielectrics. MAGMA. 2022 Apr 26. doi: 10.1007/s10334-022-01007-5. Epub ahead of print. PMID: 35471464.
3. Schmidt R, Slobozhanyuk A, Belov P, Webb A. Flexible and compact hybrid metasurfaces for enhanced ultra high field in vivo magnetic resonance imaging. Sci Rep. 2017 May 10;7(1):1678. doi: 10.1038/s41598-017-01932-9. PMID: 28490772; PMCID: PMC5431866.
4. Shchelokova A, Schmidt R, Slobozhanyuk A, Kallos T, Webb A and Belov P. A. Enhancement of magnetic resonance imaging with metasurfaces: From concept to human trials. 2017 11th International Congress on Engineered Materials Platforms for Novel Wave Phenomena (Metamaterials), 2017, pp. 31-33, doi:10.1109/MetaMaterials.2017.8107800.
5. Chen H, Guo L, Li M, Destruel A, Liu C, Weber E, Liu F, Crozier S. Metamaterial-Inspired Radiofrequency (RF) Shield With Reduced Specific Absorption Rate (SAR) and Improved Transmit Efficiency for UHF MRI. IEEE Transactions on Biomedical Engineering, vol. 68, no. 4, pp. 1178-1189, April 2021, doi: 10.1109/TBME.2020.3022884.
6. Oberacker E, Kuehne A, Oezerdem C, Nadobny J, Weihrauch M, Beck M, Zschaeck S, Diesch C, Eigentler TW, Waiczies H, Ghadjar P, Wust P, Winter L, Niendorf T. Radiofrequency applicator concepts for thermal magnetic resonance of brain tumors at 297 MHz (7.0 Tesla). Int J Hyperthermia. 2020;37(1):549-563.
7. Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3(8):487-497.
8. Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 2007;19(6):418-426.
9. Lee Titsworth W, Murad GJ, Hoh BL, Rahman M. Fighting fire with fire: the revival of thermotherapy for gliomas. Anticancer Res 2014;34(2):565-574.
10. Eigentler TW, Winter L, Han H, Oberacker E, Kuehne A, Waiczies H, Schmitter S, Boehmert L, Prinz C, Trefna HD, Niendorf T. Wideband Self-Grounded Bow-Tie Antenna for Thermal MR. NMR Biomed. 2020 May;33(5):e4274.
11. Kuehne A, Oberacker E, Waiczies H, Niendorf T. Solving the Time- and Frequency-Multiplexed Problem of Constrained Radiofrequency Induced Hyperthermia. Cancers. 2020; 12(5):1072.
12. Zanoli M, Trefná HD. Iterative time-reversal for multi-frequency hyperthermia. Phys Med Biol. 2021 Feb 11;66(4):045027. doi: 10.1088/1361-6560/abd41a. PMID: 33326945.
13. The Transverse Magnetic Mode of Wave Propagation in Rectangular and Circular Waveguides. https://resources.system-analysis.cadence.com/blog/msa2021-the-transverse-magnetic-mode-of-wave-propagation-in-rectangular-and-circular-waveguides
Figures