4387
Advanced RF antenna arrangements to improve the efficacy of Thermal Magnetic Resonance of Glioblastoma Multiforme at 7.0T, 9.4Tand 10.5T1Berlin Ultrahigh Field Facility (B.U.F.F.), Max-Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2The Charité – Universitätsmedizin, Berlin, Germany, 3MT MedTech Engineering GmbH, Berlin, Germany, 4Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max-Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
Keywords: Interventional Devices, MR-Guided Interventions, Thermal MR
This work proposes the concept of four RF applicators combining SGBT dipole and loop building block antenna array for diagnostic proton (1H) imaging and thermal intervention at 7.0T, 9.4T and 10.5T. We demonstrated the relationship between spatial arrangement of the antennas in RF applicator and the SAR profile which further related to temperature rise for targeted thermal intervention of deep-seated brain tumors. This approach of design, simulation, optimization of EM power deposition followed by calculation of the resulting temperature distribution can be adapted as a pre-routine to conduct optimization result for individual patient for further treatment planning.PURPOSE
Temperature is a critical dimension of life with diverse biological implications. Localized thermal therapy is a potent sensitizer of chemo and radiotherapy for various cancers, can facilitate targeted drug delivery using thermo-responsive nano-carriers, and is an adjunct treatment for glioblastoma multiforme (GBM) brain tumours1-7. Thermal Magnetic Resonance (ThermalMR) is a hyperthermia variant integrating anatomical and functional MRI with radio-frequency (RF) induced heating and in vivo MR thermometry, in a multipurpose RF applicator permitting supervised targeted temperature modulation1. The efficacy of ThermalMR is governed by the design, number and spatial arrangement of RF antenna building blocks, to enable uniform B1+ for diagnostic MRI, and to ensure constructive E-field focusing in the target brain region, while sparing healthy tissue. Recognizing this opportunity, we examined the applicability of advanced RF applicator configurations tailored for ThermalMR of GBM. We designed circular and elliptical RF array configurations combining compact self-grounded bow-tie (SGBT) dipole building blocks with loop elements, and evaluated these in numerical simulations with the goal to ensure B1+ uniformity for MRI of the brain at 7.0T (300 MHz), 9.4 T (400 MHz) and 10.5T (450 MHz), to ultimately improve the efficacy of RF induced thermal therapy in a virtual brain tumor patient model.METHODS
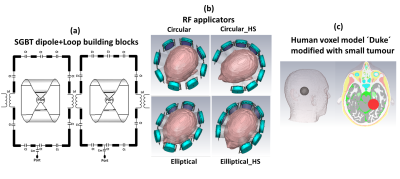
Four RF applicator configurations were investigated. Each RF applicator combines eight compact SGBT8 dipole antennas (size: 42.3x46.3x2.5mm3) with eight rectangular loop elements (size: 75x125x1mm3) (Figure 1a) to support MRI at 300MHz (B0=7.0 T), 400MHz (B0=9.4 T) and 450MHz (B0=10.5T). Circular (cir_full) and elliptical (ellip_full) RF array configurations were designed using equidistant spacing of the SGBT+loop antenna building blocks, and 3600 coverage of the antenna array around the head (Figure 1b). Additionally, horse-shoe shaped (arc= 2700 ) circular (cir_HS) and elliptical (ellip_HS) RF arrays were designed to ensure ample brain coverage while sparing the high conductivity regions of the eyes from RF exposure during targeted heating (Figure 1b). For targeted RF heating, multiple discrete frequencies (f=250, 300, 350, 400, 450MHz) were used. EMF simulations (CST Studio Suite 2020) were performed on the human voxel model ‘Duke' (IT'IS Foundation Zürich, CH)1. An intracranial sphere (radius=2cm) representing a small tumor (volume=33.5ml, σtumor=1.15S/m, εrtumor=66.5)1 was placed in the right parietal region of the brain to mimic a realistic clinical scenario (Figure 1c). Postprocessing was conducted (MATLAB 2020) to calculate B1+ fields, SAR10g and targeted heating optimization. A time- and frequency-multiplexed algorithm9 was used to provide globally optimal excitation vectors (OEV) defining the phase and amplitude setting for each RF channel. The resulting SAR distribution of the interfering incident E-fields were tailored for heating the target volume with the goal to reduce RF exposure to healthy tissues. For all RF applicators SAR10g obtained from targeted heating optimization was used to calculate temperature distribution maps of the brain with a thermal transient solver.RESULTS
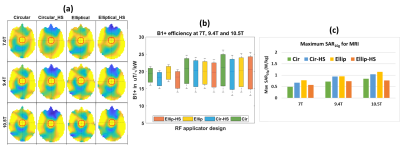
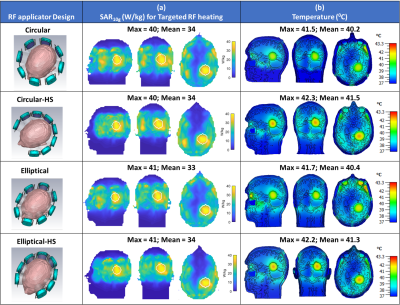
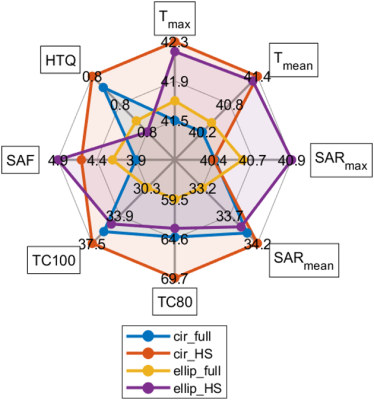
EMF simulations showed that all four hybrid RF applicators provide B1+ fields that facilitate brain MRI at 7.0T, 9.4T, 10.5T (Figure 2a,b) with maximum local SAR10g within IEC limits (Figure 2c). Results obtained for targeted RF heating and temperature treatment of the GBM model using the OEV algorithm are summarized in Figure 3. For targeted RF heating, the ellip_full and ellip_HS configurations afforded a maximum SAR10g=41W/kg, which was superior to that of the cir_full and cir_HS of maximum SAR10g=40W/kg configurations (Figure 3b). Notwithstanding the SAR10g advantage of the elliptical configurations, the horse-shoe configurations demonstrated enhanced tumor coverage10, cir_HS:TC80,TC100=70%,37.5%; ellip_HS:TC80,TC100=64%,35%, versus the 3600 cir_full and ellip_full configurations (Figure 4). The greater TC80, TC100 of the horse-shoe configurations yielded a higher temperature increase (cir_HS: ΔT=5.30C, ellip_HS:ΔT=5.20C (Figure 3c,4) from 370C basal body temperature to 42.30C and 42.20C, versus the cir_full and ellip_full configurations for a heating regime of ~40 W/kg SAR10g. The horse-shoe configurations can improve patient comfort and reduce RF power deposition in the facial orbit. Owing to the design, both horse-shoe configurations exhibited a higher SAR amplification factor10 (SAF) and lower hotspots to quotients10 (HTQ) compared to the 3600 configurations (Figure 4). Closer examination of the horse-shoe configurations revealed that the cir_HS configuration facilitated a slightly higher temperature rise versus the ellip_HS configuration. This is due to the increased TC100 of the cir_HS configuration. Notwithstanding this minor maximum advantage of the cir_HS configuration, the ellip_HS configuration demonstrated higher SAF and lower HTQ than the cir_HS design. In conclusion, ellip_HS performed better than cir_HS because healthy remote tissue was better saved from unwanted SAR and temperature hotspots due to higher SAF and lower HTQ.DISCUSSIONS & CONCLUSIONS
We show that all four RF applicators are suited for MRI at 7.0T, 9.4T and 10.5T, without changing the antenna geometry, given the multi-resonant broadband characteristics of the SGBT+loop building blocks. We demonstrate that the horse-shoe configurations outperform the 3600 configurations for ThermalMR of the virtual GBM patient setup. The enhanced RF power deposition in the target volume and the temperature rise up to ~42.30C inside the tumor target volume bodes well for the needs of hyperthermia therapy of GBM. Our EMF and temperature simulations build a technical foundation for the implementation and application of the elliptical horse-shoe RF configuration and provide springboard for the development and optimization of novel RF applicators tailored for ThermalMR based therapy of brain tumours.Acknowledgements
This project was funded in part by an advanced ERC grant (EU project Thermal MR: 743077).References
1. Oberacker E, Kuehne A, Oezerdem C, Nadobny J, Weihrauch M, Beck M, Zschaeck S, Diesch C, Eigentler TW, Waiczies H, Ghadjar P, Wust P, Winter L, Niendorf T. Radiofrequency applicator concepts for thermal magnetic resonance of brain tumors at 297 MHz (7.0 Tesla). Int J Hyperthermia. 2020;37(1):549-563.
2. Ji Y, Winter L, Navarro L, Ku MC, Periquito JS, Pham M, Hoffmann W, Theune LE, Calderón M, Niendorf T. Controlled Release of Therapeutics from Thermoresponsive Nanogels: A Thermal Magnetic Resonance Feasibility Study. Cancers (Basel). 2020 May 27;12(6):1380.
3. Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3(8):487-497.
4. Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner CM. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol 2010;11(6):561-570.
5. Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 2007;19(6):418-426.
6. Lee Titsworth W, Murad GJ, Hoh BL, Rahman M. Fighting fire with fire: the revival of thermotherapy for gliomas. Anticancer Res 2014;34(2):565-574.
7. Restivo MC, van den Berg CAT, van Lier ALHMW, et al. Local specific absorption rate in brain tumors at 7 tesla. Magn Reson Med.2016;75(1):381–389.
8. Eigentler TW, Winter L, Han H, Oberacker E, Kuehne A, Waiczies H, Schmitter S, Boehmert L, Prinz C, Trefna HD, Niendorf T. Wideband Self-Grounded Bow-Tie Antenna for Thermal MR. NMR Biomed. 2020 May;33(5):e4274.
9. Kuehne A, Oberacker E, Waiczies H, Niendorf T. Solving the Time- and Frequency-Multiplexed Problem of Constrained Radiofrequency Induced Hyperthermia. Cancers. 2020; 12(5):1072.
10. Zanoli M, Trefná HD. Iterative time-reversal for multi-frequency hyperthermia. Phys Med Biol. 2021 Feb 11;66(4):045027. doi: 10.1088/1361-6560/abd41a. PMID: 33326945.
11. Omer I, Erdal K, Bahattin T. Antenna Arrangement Considerations for Microwave Hyperthermia Applications. Internal union of radio science, proceedings of the 75th URSI Anniversary Symposium, URSI General Assembly 2011, Istanbul, Turkey
12. Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med.1990 Nov;16(2):192-225
Figures