4341
Towards Biomedical Applications of Parahydrogen Induced Polarization by Side-Arm Hydrogenation Using SAMBADENA
Henri de Maissin1,2, Obaid Mohiuddin1, Marvin Herzog1, Eduard Y. Chekmenev3,4, Sergey Korchak5,6, Stefan Glöggler5,6, Jan-Bernd Hövener7, Maxim Zaitsev1, Dominik v. Elverfeldt1, and Andreas B. Schmidt1,3,8
1Division of Medical Physics, University Medical Center Freiburg, Freiburg im Breisgau, Germany, 2German Cancer Research Center (DKFZ), German Cancer Consortium (DKTK), Heidelberg, Germany, 3Integrative biosciences (Ibio), department of Chemistry, Wayne state university, Detroit, MI, United States, 4Russian Academy of Sciences (RAS), Moscow, Russian Federation, 5NMR Signal Enhancement Group, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany, 6Center for Biostructural Imaging of Neurodegeneration, University Medical Center Göttingen, Göttingen, Germany, 7Section Biomedical Imaging, Molecular Imaging North Competence Center, Kiel, Germany, 8German Cancer Consortium (DKTK), Heidelberg, Germany
1Division of Medical Physics, University Medical Center Freiburg, Freiburg im Breisgau, Germany, 2German Cancer Research Center (DKFZ), German Cancer Consortium (DKTK), Heidelberg, Germany, 3Integrative biosciences (Ibio), department of Chemistry, Wayne state university, Detroit, MI, United States, 4Russian Academy of Sciences (RAS), Moscow, Russian Federation, 5NMR Signal Enhancement Group, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany, 6Center for Biostructural Imaging of Neurodegeneration, University Medical Center Göttingen, Göttingen, Germany, 7Section Biomedical Imaging, Molecular Imaging North Competence Center, Kiel, Germany, 8German Cancer Consortium (DKTK), Heidelberg, Germany
Synopsis
Keywords: High-Field MRI, Hyperpolarized MR (Non-Gas), parahydrogen cancer spectroscopy
Parahydrogen-induced Polarization (PHIP) by Synthesis Amid the Magnet Bore Allows Dramatically Enhanced Nuclear Alignment (SAMBADENA) of agents in situ within the MRI magnet and requires very little hardware in addition to the MRI system. However, side-arm hydrogenation (PHIP-SAH), fast side-arm cleavage and purification have not been attempted yet.Here, we present a SAMBADENA setup made from commercially-available components for future preclinical applications. Using this setup, we demonstrate high 13C polarizations of 17% for 25mM ethyl-[1-13C]-acetate. In addition, for ethyl-pyruvate, fast side-arm cleavage and removal of 96% of the organic solvent within 10s to obtain pyruvate in neat water is demonstrated.
INTRODUCTION
Hyperpolarized (HP) 13C MRI has enabled unprecedented real-time imaging of metabolism in patients with great potential for diagnostics and personalized therapy1,2. Parahydrogen (pH2) induced polarization (PHIP) by side-arm hydrogenation (PHIP-SAH) has opened a low-cost, high-throughput pathway to produce hyperpolarized metabolites3,4. Recently, we demonstrated Synthesis Amid the Magnet Bore that Allowed a Dramatically Enhanced Nuclear Alignment (SAMBADENA)5. Using this approach, PHIP agents have been produced quasi-continuously in situ inside the MRI system every 15s and fast administration and 13C-imaging in vivo within 15s after hyperpolarization has been demonstrated6,7. However, the purification to obtain neat aqueous metabolite solutions has not been attempted yet.METHODS
Setup: The SAMBADENA setup comprised a preclinical 7T MRI system, 1H-13C volume coil for excitation, 13C receive surface coil, and the pH2-bubbling setup (Fig. 1). The latter comprised two gas cylinders (N2 and pH2), pressure regulators, a digital manometer, needle valves to regulate gas-flow, tubing and fittings, magnetic valves to gate the flow of gases, a custom power-relay box to control the valve actuation from the MRI pulse program, and a standard 5-mm NMR tube to host the hydrogenation reaction. An ultra-fine 1/32” PEEK catheter was used to guide pH2 to the bottom of the NMR tube and through the reaction solution, to reduce magnetic field inhomogeneities8. The NMR tube was centered in the MRI system using a custom 3D-printed holder (Fig. 1A). Apart from the holder and power relay, all materials are commercially available (Table 1). Total cost of the pH2-bubbling system is ≈1000€.Samples: pH2 was enriched to ≈85% using a helium-based cryostat and an iron-oxide catalyst9. For the reaction solutions, 25mM vinyl-[1-13C]-acetate-d6 (VA, 1.1% naturally abundant 13C; CAS: 189765-98-8) and 3mM rhodium-based hydrogenation catalyst ([Rh(dppb)(COD)]BF4, CAS: 79255-71-3) were mixed in acetone-d6 (CAS: 666-52-4) and oxygen was removed by guiding N2 through the solution for 1min prior to hydrogenation.
Hyperpolarization: 200µL of this solution was filled into the NMR tube, which was then connected to the pH2-bubbling system. The hyperpolarization pulse program was started and the NMR tube was back-pressurized with 2.5bar N2 to prevent evaporation before heating. The sample was heated for 20s in an ≈80°C water bath. Subsequently, the tube was positioned at the MRI isocenter and a manual trigger button was actuated to start the hydrogenation of the precursor (Fig. 1B): 8bar pH2 were guided through the sample for 12s under continuous-gas-flow condition. Next, the NMR tube was bypassed for 1s to stop the pH2 flow and the spin-order transfer (SOT) from pH2-derived protons to the [1-13C]-acetate nucleus was conducted (ESOTHERIC sequence10, (Fig. 1C), total length of 388ms). Hyperpolarized 13C signal was detected at the end of SOT and quantified by comparing it with the signal of a thermally-polarized reference solution (4M [1-13C]sodium acetate (CAS:23424-28-4) in 250µL H2O).
Hydrolysis and purification: Similar to a recent report11, the NMR tube connected to the SAMBADEMA setup was filled with a thermally-polarized 400µL solution of 50mM ethyl pyruvate (CAS:617-35-6) in acetone-d6. Note that ethyl pyruvate was used here instead of ethyl acetate used for the HP experiments. Note that the precursor was not 13C-enriched. To perform side-arm cleavage, 200µL of 150mM sodium carbonate (CAS:144-55-8) in D2O (CAS:7789-20-0) was injected into the tube and mixed with N2 for 10s. The tube was then exposed to vacuum while being heated in a 50°C water bath to remove acetone from the solution. The vacuum pump was connected to the SAMBADENA near the outlet. (Fig. 1A) NMR spectra of the solution were acquired after each of these steps using a 1T benchtop NMR spectrometer (43MHz, Magritek) and integrated with a custom routine (Matlab R2020a, MathWorks, USA). (Fig. 3)
RESULTS
Using this setup made from commercially-available materials, 13C polarization of 16.7% was achieved for 25mM ethyl-acetate-d6 (EA), the hydrogenation product of VA (Fig. 2). With the described protocol for hydrolysis and purification, fast side-arm cleavage was observed (<10s). Only 4.3% of the final solution volume was acetone-d6, and 56.6% of the starting amount of pyruvate was successfully reconstituted in D2O. (Fig. 3).DISCUSSION
The observed 13C polarizations are already high and can likely be improved considering that >50% has been demonstrated for ethyl-[1-13C]-acetate using an NMR setup12. A higher pH2 enrichment and faster hydrogenation, which seems feasible by increasing the pH2 flow and temperature during the reaction, are expected to be constructive. Also, it has been shown that SOT in the MRI system can be compromised by B0 inhomogeneity and B1 1H-excitation bandwidth8,13. Hence, more sophisticated RF pulse schemes like composite or adiabatic pulses should be investigated14. The successful extraction of purified pyruvate in water is most promising and next needs to be refined for faster purification procedure and removal of hydrogenation catalyst.CONCLUSION
For the first time, SAMBADENA was demonstrated using mostly commercially available parts. While polarization and agent concentration can likely be improved, they are already sufficient for preclinical studies. By applying the cleavage and purification protocol to hyperpolarized samples, first pilot biomedical applications using SAMBADENA appear to be on the horizon15. This technique and setup are available to many groups with the promise to accelerate the translation of PHIP and wide use of HP metabolic MRI.Acknowledgements
This work was supported by the German Cancer Consortium (DKTK), B.E.S.T. Fluidsysteme GmbH I Swagelok Stuttgart, the German Research Foundation (SCHM 3694/1-1, SCHM 3694/2-1, SFB1479, HO-4604/2, HO-4604/3), and the Federal Ministry of Education and Research (BMBF) lighthouse project QuE-MRT and Juniorverbund 01ZX1915C (AP). ABS and EYC thank Wayne State University for Postdoctoral Fellow award.References
1. Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019;21:1–16 doi: 10.1016/j.neo.2018.09.006.2. Chowdhury R, Mueller CA, Smith L, et al. Quantification of Prostate Cancer Metabolism Using 3D Multiecho bSSFP and Hyperpolarized [1-13C] Pyruvate: Metabolism Differs Between Tumors of the Same Gleason Grade. J. Magn. Reson. Imaging n/a doi: 10.1002/jmri.28467.
3. Reineri F, Boi T, Aime S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat. Commun. 2015;6:5858 doi: 10.1038/ncomms6858.
4. Schmidt AB, Bowers CR, Buckenmaier K, et al. Instrumentation for Hydrogenative Parahydrogen-Based Hyperpolarization Techniques. Anal. Chem. 2022;94:479–502 doi: 10.1021/acs.analchem.1c04863.
5. Schmidt AB, Berner S, Schimpf W, et al. Liquid-state carbon-13 hyperpolarization generated in an MRI system for fast imaging. Nat. Commun. 2017;8:14535 doi: 10.1038/ncomms14535.
6. Schmidt AB, Zimmermann M, Berner S, et al. Quasi-continuous production of highly hyperpolarized carbon-13 contrast agents every 15 seconds within an MRI system. Commun. Chem. 2022;5:1–7 doi: 10.1038/s42004-022-00634-2.
7. Schmidt AB, Berner S, Braig M, et al. In vivo 13C-MRI using SAMBADENA. PLOS ONE 2018;13:e0200141 doi: 10.1371/journal.pone.0200141.
8. Ivantaev V, Berner S, de Maissin H, et al. Molecular translation in inhomogeneous field can dramatically reduce the efficiency of spin-order transfer at high field. In: Proc. Intl. Soc. Mag. Reson. Med. 29. Online; 2021. p. 3798.
9. Hoevener J-B, Bär S, Leupold J, et al. A continuous-flow, high-throughput, high-pressure parahydrogen converter for hyperpolarization in a clinical setting. NMR Biomed. 2013;26:124–131.
10. Korchak S, Yang S, Mamone S, Glöggler S. Pulsed Magnetic Resonance to Signal-Enhance Metabolites within Seconds by utilizing para-Hydrogen. ChemistryOpen 2018;7:344–348 doi: 10.1002/open.201800024.
11. Ding Y, Korchak S, Mamone S, et al. Rapidly Signal-enhanced Metabolites for Atomic Scale Monitoring of Living Cells with Magnetic Resonance. Chemistry–Methods n/a:e202200023 doi: 10.1002/cmtd.202200023.
12. Korchak S, Mamone S, Glöggler S. Over 50 % 1H and 13C Polarization for Generating Hyperpolarized Metabolites—A para-Hydrogen Approach. ChemistryOpen 2018;7:672–676 doi: 10.1002/open.201800086.
13. de Maissin H, Berner S, Ivantaev V, et al. Dramatic Effect of Pulse Length and Bandwidth on the Efficiency of Pulsed Spin-Order-Transfer Sequences at High Field. In: Proc. Intl. Soc. Mag. Reson. Med. 29. Online; 2021. p. 3805.
14. Pravdivtsev AN, Hövener J-B, Schmidt AB. Frequency-Selective Manipulations of Spins allow Effective and Robust Transfer of Spin Order from Parahydrogen to Heteronuclei in Weakly-Coupled Spin Systems. ChemPhysChem 2022;23:e202100721 doi: 10.1002/cphc.202100721.
15. Hune T, Mamone S, Schroeder H, et al. Metabolic Tumor Imaging with Rapidly Signal-Enhanced 1-13C-Pyruvate-d3. ChemPhysChem n/a doi: 10.1002/cphc.202200615.
Figures
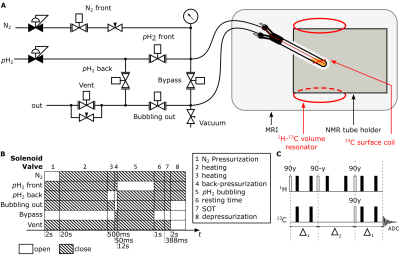
Figure 1: Schematic of the SAMBADENA setup (A), experimental procedure (B), and the used spin-order-transfer sequence ESOTHERIC (Δ1=158ms, Δ2=78ms) (C). The 1H-13C volume resonator produces a homogenous B1 excitation field, while the 13C surface coil loop placed around the NMR tube improves sensitivity for detection of 13C signal. This fully automated solenoid valve actuation scheme allows for a controlled hydrogenation and avoids pH2-bubbling before the 12s time interval.
Table 1: List of the used, commercially available components (A), and chemicals (B). All the components are commercially available but the NMR tube holder, which was 3D-printed.
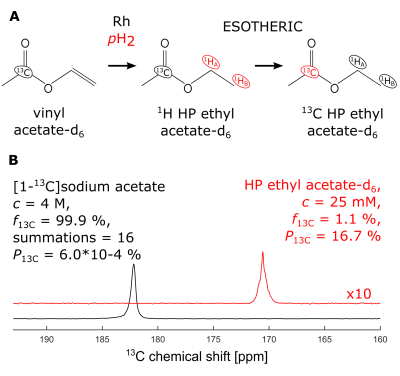
Figure 2: Hyperpolarization of ethyl-[1-13C]-acetate. (A) Upon catalytic addition of parahydrogen (pH2), vinyl-acetate-d6 forms ethyl-acetate-d6. The pH2-derived spin order (red) is then transferred to the [1-13C] nucleus of acetate. (B) 13C NMR spectra of hyperpolarized ethyl-[1-13C]-acetate-d6 and a highly concentrated, 13C-enriched sample of sodium acetate (the reference solution was 250µL while the HP solution was 200µL). Note that the vinyl acetate-d6 used here was not 13C-enriched.
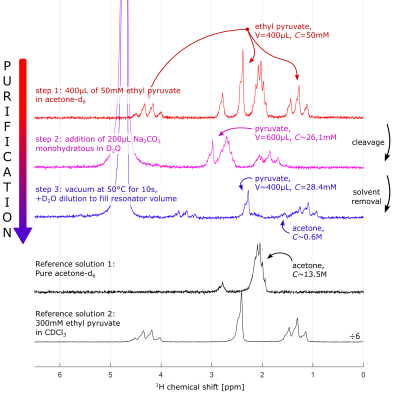
Figure 3: 1H-NMR spectra acquired before and after side-arm cleavage and purification. Solutions were measured at each of the described procedures as well as two reference solutions using 1T benchtop NMR. The solution in step 1 contains 20µmole of pyruvate while the solution in step 3 has 11.3µmole. The total volume contribution of acetone in the solution at step 3 is 4.5%.
DOI: https://doi.org/10.58530/2023/4341