4339
GRAPPA-Accelerated Flyback EPI with Controlled Aliasing for Hyperpolarized Pyruvate1Electrical Engineering, UT Dallas, Richardson, TX, United States, 2Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 3GE Healthcare, New York, NY, United States, 4Department of Radiology, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Keywords: Image Reconstruction, Phantoms
In this study, we introduced the parallel imaging technique GRAPPA to the flyback EPI with controlled aliasing in order to accelerate the acquisition time while simultaneously reducing artifacts. The GRAPPA-accelerated flyback EPI was characterized using MR phantoms with varying SNR and acceleration factor (R) and the feasibility of simultaneous bicarbonate and lactate imaging with the proposed scheme was also demonstrated. The proposed acquisition method has potential advantages for in vivo studies with hyperpolarized substrates by shortening acquisition time, improving spatial resolution, and increasing effective flip angles.Introduction
Metabolic imaging with hyperpolarized substrates requires rapid acquisition of multiple metabolites to capture the time-varying distributions. Flyback EPI in combination with a spectral-spatial RF pulse is a robust imaging option for metabolite-interleaved acquisition, particularly for 13C imaging that is difficult to perform proper refence scans. As flyback EPI is susceptible to off-resonance, shifting images along the phase encoding direction, simultaneous imaging of two hyperpolarized metabolites using a controlled aliasing scheme by modulating the echo-spacing was suggested (1,2). In this study, we introduced the parallel imaging technique GeneRalized Autocalibrating Partial Parallel Acquisition (GRAPPA) to the flyback EPI scheme with controlled acquisition in order to accelerate the acquisition time while simultaneously reducing artifacts.Methods
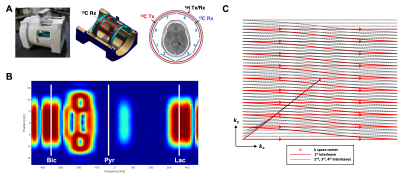
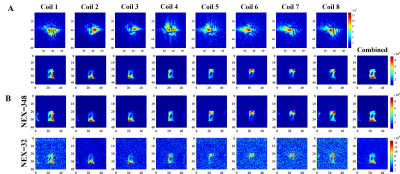
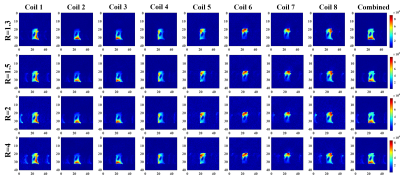
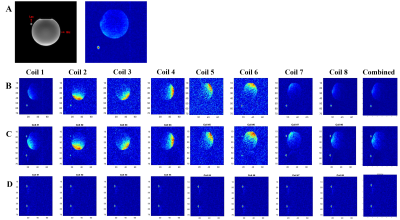
A 13C/1H dual-frequency RF head coil that consists of 1H quadrature transceiver, 13C quadrature transmit and 8-channel 13C receive arrays (Clinical MR Solutions, LLC) was used for the study (3), Figure 1A. For simultaneous excitation of two major products, [13C]bicarbonate and [1-13C]lactate, of hyperpolarized [1-13C]pyruvate, a spectral-spatial RF pulse that selectively excites both lactate and bicarbonate was used (1), Figure 1B. Spatially-interleaved flyback EPI readout was used for to acquire fully sampled k-space data, Figure 1C. The echo-spacing was calculated in order to completely separate the lactate and bicarbonate images along the phase encoding direction (1,2). The GRAPPA-accelerated flyback EPI was characterized using a gadolinium-doped (1mM Prohance) 0.4-M [13C]bicarbonate phantom (Figure 1D) by retrospectively varying SNR and acceleration factor (R) from a dataset acquired using the flyback EPI (TE/TR = 8 us/5000 ms, thickness = 20 mm, matrix size = 118 x 128, FOV = 36 x 36 cm2, #averages = 348, #interleaves = 4). To demonstrate the feasibility of simultaneous bicarbonate and lactate imaging with the proposed scheme, two phantoms consisting of a 0.4-M [13C]bicarbonate sphere (diameter = 18 cm) and a 6-M [1-13C]lactate cylinder (diameter = 1 cm) were used and retrospectively undersampled along the phase-encoding direction (R = 2). All the data were acquired using a GE 3T 750W wide-bore scanner.Results and Discussion
As expected, the artifacts associated with the GRAPPA acceleration increased as the image SNR decreased, Figure 2, and as the acceleration factor increased, Figure 3. When applied to the lactate-bicarbonate phantoms with R = 2, the lactate image created an alias unlike the bicarbonate image. The coil elements that are located far from the lactate phantom (e.g., channels #4-8) could not detect the lactate signal primarily due to the small size and the partial localization of the phantom, Figure 4. The proposed acquisition method has several advantages for in vivo studies with hyperpolarized substrates. First, the scan time per image cab be reduced, minimizing the risk of motion artifacts and improving the point spread function. Second, the number of interleaves can be reduced. The smaller number of interleaves will increase the effective flip angle per interleave and hence the SNR. We may obtain both lactate and bicarbonate signals in one excitation depending on the hardware performance and the imaging parameters. Alternatively, the proposed scheme can be used to increase the spatial resolution of the images without additional scan time. Several other parallel imaging techniques were previously demonstrated for hyperpolarized 13C MRI by using fiducial markers (4) calibration-integrated (5–7) or calibrationless (7–9) approaches. Although GRAPPA requires no 13C coil sensitivity maps and is ideally suited for 13C MRI, it has not been actively exploited so far. As the GRAPPA only requires simple modification in the readout gradients, we expect the acceleration scheme can be readily extended to other imaging-based acquisition methods with spectra-spatial RF pulses. Future studies include designing the optimal flyback readout gradients for accelerated data acquisition.Conclusion
We demonstrated that the simultaneous imaging of hyperpolarized lactate and bicarbonate using the flyback EPI with controlled aliasing scheme can be accelerated by undersampling k-space along the phase-encoding direction and the GRAPPA reconstruction.Acknowledgements
National Institute of Health: R01NS107409, R21EB030765, P30DK127984, P41EB015908; Department of Defense: W81XWH2210485; The Welch Foundation: I-2009-20190330References
1. Ma J, Hackett EP, Schulte RF, Park JM. Simultaneous Assessment of Hyperpolarized [1-13C] Lactate and [13C] Bicarbonate with Fly-Back Echo Planar Imaging. In: International Society of Magnetic Resonance in Medicine. 2019. #1949.
2. Reed GD, Larson PEZ, Morze C Von, Bok R, Lustig M, Kerr AB, et al. A method for simultaneous echo planar imaging of hyperpolarized 13C pyruvate and 13C lactate. J Magn Reson [Internet]. 2012;217:41–7. Available from: http://dx.doi.org/10.1016/j.jmr.2012.02.008
3. Ma J, Pinho MC, Harrison CE, Chen J, Sun C, Hackett EP, et al. Dynamic 13C MR spectroscopy as an alternative to imaging for assessing cerebral metabolism using hyperpolarized pyruvate in humans. Magn Reson Med. 2022;87(3):1136–49.
4. Lau AZ, Lau JYC, Chen AP, Cunningham CH. Simultaneous multislice acquisition without trajectory modification for hyperpolarized 13C experiments. Magn Reson Med. 2018;80(4):1588–94.
5. Arunachalam A, Whitt D, Fish K, Giaquinto R, Piel J, Watkins R, et al. Accelerated spectroscopic imaging of hyperpolarized C-13 pyruvate using SENSE parallel imaging. NMR Biomed. 2009;22(8):867–73.
6. Ohliger MA, Larson PEZ, Bok RA, Shin P, Hu S, Tropp J, et al. Combined parallel and partial fourier MR reconstruction for accelerated 8-channel hyperpolarized carbon-13 in vivo magnetic resonance Spectroscopic imaging (MRSI). J Magn Reson Imaging. 2013;38(3):701–13.
7. Feng Y, Gordon JW, Shin PJ, Von Morze C, Lustig M, Larson PEZ, et al. Development and testing of hyperpolarized 13C MR calibrationless parallel imaging. J Magn Reson [Internet]. 2016;262:1–7. Available from: http://dx.doi.org/10.1016/j.jmr.2015.10.018
8. Shin PJ, Larson PEZ, Ohliger MA, Elad M, Pauly JM, Vigneron DB, et al. Calibrationless parallel imaging reconstruction based on structured low-rank matrix completion. In: Magnetic Resonance in Medicine. 2014. p. 959–70.
9. Gordon JW, Hansen RB, Shin PJ, Feng Y, Vigneron DB, Larson PEZ. 3D hyperpolarized C-13 EPI with calibrationless parallel imaging. J Magn Reson [Internet]. 2018;289:92–9. Available from: https://doi.org/10.1016/j.jmr.2018.02.011
Figures