4337
Increasing the hyperpolarization level on PHIP-SAH polarized pyruvate in a fully biocompatible solution.1Molecular Biotechnology and Health Sciences, University of Torino, Torino, Italy, 2National Research Council, Torino, Italy
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Hyperpolarized MR (Non-Gas), metabolism
Hyperpolarized [1-13C]pyruvate can be obtained by means of ParaHydrogen Induced Polarization-Side Arm Hydrogenation, a hyperpolarization technique that is significantly more cost effective and faster than the gold-standard method d-DNP. The hyperpolarization level that we reported previously, for the fully biocompatible aqueous solution of this HP metabolite, was sufficient to carry out metabolic studies, but still low. In this work we show that the use of a lower catalyst concentration, together with a co-catalyst, and an improved magnetic field cycle profile for spin order transfer can double the HP level on this metabolite.Introduction
The parahydrogen based method named PHIP-SAH (Side Arm Hydrogenation) allows to obtain hyperpolarized pyruvate [1] and other metabolites, at a fraction of the costs given by the application of the d-DNP instrumentation and with faster hyperpolarization cycles. A few metabolic studies using pyruvate hyperpolarized by means of this technique have already been reported [2], nevertheless the polarization level reported on the fully biocompatible aqueous solution was only around 2.5% [3]. In the herein reported work, two passages of the procedure, the hydrogenation reaction and the spin order transfer, have been modidies, leading to an increased hyperpolarization level.Methods
In the PHIP-SAH procedure, an unsaturated ester derivative of pyruvate (propargyl-[1-13C]pyruvate) is hydrogenated, using hydrogen enriched in the para-isomer (para-H2) and a metal complex, in chloroform. While in the previously reported work, the catalyst-to-substrate ratio was 1/20, in the herein reported work, the catalyst concentration has been reduced more than five times, while keeping the same efficiency in terms of yield and speed of the hydrogenation reaction. This has been achieved thanks to the addition of a ligand in the hydrogenation mixture that acts as an adjuvant of the metal complex. After completion of the reaction, spin order transfer from parahydrogen protons to 13C must occur, in order to obtain net hyperpolarization on the heteroatom. This has been accomplished through the application of magnetic field cycling [4]. According to this method, the magnetic field in which the parahydrogenated sample is placed, is suddenly dropped from a few micro-T to a few nano-T and then adiabatically increased, in a controlled way, using a controlled electric current. The MFC setup is made of a magnetic field shield (triple shield mu-metal box, Bartington Instruments) equipped with a coil fed with controlled current provided by an arbitrary wave generator. In these experiments, a tailored remagnetization passage has been applied, instead of the exponential remagnetization used in the previously reported experiments.Results
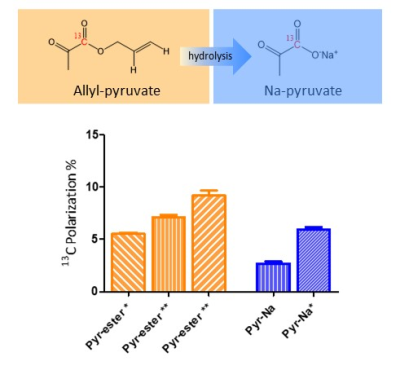
The significant reduction of the catalyst concentration, together with the application of a tailored MFC procedure, lead to an increase of the hyperpolarization level on the 13C signal of allyl-[1-13C]pyruvate from the previously reported 5.2±0.6% to 9.2 ±1.0 % (corresponding to a signal enhancement 7800±832, for 13C at 14.1T). Following to hydrolysis and phase extraction, the aqueous solution of hyperpolarized sodium [1-13C]pyruvate has been diluted with H2O (1:1=aqueous phase:H2O) and in-vitro 13C-MRI has been carried out (figure 2). A sequence of five single shot 13C-FLASH images has been acquired, using a small flip angle pulse (15°pulse) and repetition time 3s.The hyperpolarization level on the 13C-carboxylate of sodium [1-13C]pyruvate has been measured, in a separate set of experiments, and is 5.9±0.5%, being the previously reported value 2.7±0.6%.Discussion and conclusions
A decrease of the catalyst concentration can be valuable per-se, since it leads to further decrease the of metal traces in the final solution. The herein reported experiments show that a lower catalyst-to-substrate ratio allows to increase the polarization level on the product. It can also be observed that hyperpolarization losses during hydrolysis are reduced with respect to what reported previously [3]. In the herein reported experiments, about 60% of the 13C hyperpolarization observed on the ester is maintained on sodium pyruvate, while, in the work carried out using the higher catalyst concentration, only about 40% of the ester hyperpolarization was kept on the hydrolysis product. This demonstrates that the catalyst plays a relevant role in determining polarization losses during the first step of the hyperpolarization procedure, and different complexes will be investigated in future works.Acknowledgements
EU is gratefuly acknowledged for funding (H2020 FETOPEN 2018-2020, No. 858149, AlternativesToGd).References
[1] ParaHydrogen Induced Polarization of 13C carboxylate resonance in Acetate and pyruvate” Reineri F., Boi T., Aime S., Nat. Commun. 2015, 6:5858. 10.1038/ncomms6858.
[2]The 13C hyperpolarized pyruvate generated by ParaHydrogen detects the response of the heart to altered metabolism in real time. E. Cavallari, C. Carrera, M. Sorge, G. Bonne, A. Muchir, S. Aime, F. Reineri Scientific Reports, 2018, 8:8366 DOI:10.1038/s41598-018-26583-2.
[3] Effect of the hydrogenation solvent in the PHIP-SAH hyperpolarization of [1-13C]pyruvate” Bondar O., Cavallari E., Carrera C., Aime S., Reineri F. (2022) Catalysis Today, 397-399, pp. 94 - 102, Cited 1 times. DOI: 10.1016/j.cattod.2021.11.03.
[4]Transfer of para-hydrogen spin order into polarizationby diabatic field cycling. H. Johannesson, O. Axelsson, M. Karlsson. C. R. Physique 5 (2004) 315–324. doi:10.1016/j.crhy.2004.02.001
Figures