4334
Para-hydrogen signal-enhanced 1-13C-pyruvate-d3 for rapid metabolic tumor imaging
Henning Schroeder1, Theresa Hune1, Salvatore Mamone1, Anil Jagtap1, Sonja Sternkopf1, Gabriele Stevanato1, Sergey Korchak1, Claudia Fokken2, Christoph Müller3,4, Dorothea Becker2, Andreas Schmidt3,4,5, and Stefan Glöggler1
1NMR Signal Enhancement, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany, 2Department of NMR-based Structural Biology, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany, 3Division of Medical Physics, Department of Radiology, Medical Center, University of Freiburg, Freiburg, Germany, 4German Cancer Consortium (DKTK), partner site Freiburg, German Cancer Research Center (DKFZ), Heidelberg, Germany, 5Intergrative Biosciences (Ibio), Department of Chemistry, Karmanos Cancer Institute (KCI), Wayne State University, Detroit, MI, United States
1NMR Signal Enhancement, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany, 2Department of NMR-based Structural Biology, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany, 3Division of Medical Physics, Department of Radiology, Medical Center, University of Freiburg, Freiburg, Germany, 4German Cancer Consortium (DKTK), partner site Freiburg, German Cancer Research Center (DKFZ), Heidelberg, Germany, 5Intergrative Biosciences (Ibio), Department of Chemistry, Karmanos Cancer Institute (KCI), Wayne State University, Detroit, MI, United States
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Cancer
Using a Parahydrogen-induced polarization (PHIP) based approach, we enhanced the signal of pyruvate, one of the key metabolites for energy production in the body. By injecting this metabolite into a mouse carrying a human melanoma tumor, we could perform real time spectroscopy and visualization of the tumors metabolism using a NMR machine. This offers a fast and powerful tool for metabolic imaging without using radio tracers.INTRODUCTION
Changes in metabolism are often the first signs of pathological changes in tissues and therefore have a high diagnostic value. Metabolites could serve as efficient biomarkers, however for the application in MRI requires a boost in sensitivity since the baseline signals are not strong enough to be detected. Using para-hydrogen to enhance NMR signals is a convenient and fast way to overcome this limitation. [1,2] Via hydrogenation of a removable side-arm, this technique is now applicable to a wide range of molecules. [3] High signal intensities enable techniques like Magnetic Resonance Spectroscopic Imaging (MRSI) to trace metabolic changes, for example in potential tumors, in vivo. [4] The use of 13C-pyruvate has been shown to improve on the imaging sensitivity of cancer imaging approaches such as Positron Emission Tomography (PET) at least in specific uses [5].METHODS
In this work, 13C signal-enhanced (SE) 1-13C-pyruvate obtained by following the MINERVA protocol was used to investigate cancer metabolism in mouse models. [6] Crucial steps are the hydrogenation of vinyl pyruvate using para-enriched hydrogen and the nuclear polarization transfer to the carbon of interest that result in more than 50% hyperpolarization. Bond cleavage and catalyst filtering lead to a clean aqueous solution with a concentration of 50 mM of the SE metabolite. As a model, subcutaneous human melanoma xenografts above the flanks in Balb/c nu/nu mice were chosen. The SE pyruvate was injected into the tail vein of the mice resulting in the same blood concentrations used in human studies (2 mM) and time-resolved NMR spectra as well as metabolic images using an EPSI sequence were acquired.RESULTS
Cancer in general, and the chosen model in particular, shows very rapid growth and has therefore a high demand for energy. This leads to an increased conversion of pyruvate to lactate within the tumor, known as the Warburg effect. This was evident in the NMR spectra we recorded. Pyruvate is rapidly converted into lactate post injection into the bloodstream (see Fig. 1). The concentration profiles were fitted using a model-free approach based on the area under the curves. From that, the rate constant of the conversion from pyruvate to lactate was determined. For the metabolic images, a 2D-EPSI sequence (Echo Planar Spectroscopic Imaging) was used. The resolution was 3 x 3 mm2. The images show strong pyruvate and lactate signals at the site of the tumor (see Fig. 2), reflecting its high energy consumption and demonstrating the feasibility of this method for cancer imaging and diagnostics. [7] The whole procedure from the start of signal enhancement to data acquisition takes two minutes.SUMMARY
The presented combination of signal enhancement with para-hydrogen and metabolic imaging can be used to characterize tumor metabolism. Since the scope of potential biomarker applications is very large, this technique can be applied to other tumor models and a large variety of further diseases. The short preparation time of the signal-enhanced 13C-pyruvate with this method overcomes speed limitation of comparable approaches and enables a high throughput, making the technique feasible for in vivo studies and a potential use for medical imaging in the future.Acknowledgements
No acknowledgement found.References
[1] C. R. Bowers, D. P. Weitekamp, Phys. Rev. Lett. 1986, 57, 2645. [2] C. R. Bowers, D. P. Weitekamp, J. Am. Chem. Soc. 1987, 109, 5541. [3] F. Reineri, T. Boi, S. Aime, Nat.Commun. 2015, 6, 5858. [4] E. Cavallari, C. Carrera, M. Sorge, G. Bonne, A. Muchir, S. Aime, F. Reineri, Sci. Rep. 2018, 8, 8366. [5] R. Hesketh, J. Wang, A. Wright, D. Lewis, A. Denton, R. Grenfell, J. Miller, R. Bielik, M. Gehrung, M. Fala, S. Ros, B. Xie, D. Hu, K. Brindle, Cancer Res. 2019, 14, 3557 [6] Y. Ding et al., Chemistry-Methods 2022, e202200023. [7] T. Hune, S. Mamone, H. Schroeder et al., ChemPhysChem. 2022, Accepted Author Manuscript. https://doi.org/10.1002/cphc.202200615Figures
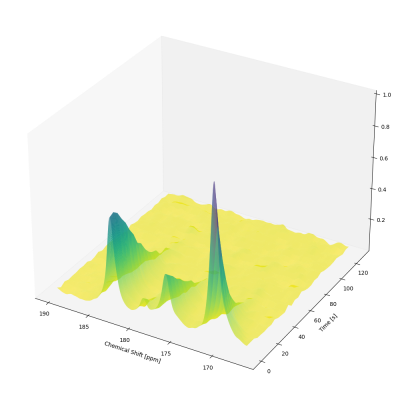
Fig. 1: Waterfall plot depicting the NMR spectra starting 16 seconds post injection. The conversion of pyruvate (right) into alanine (middle) and lactate (left) is shown over time.
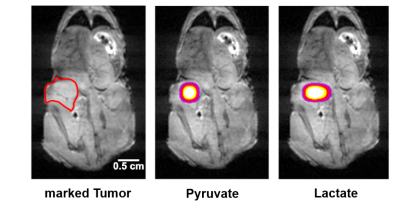
Fig. 2: MRI image (hydrogen) of a mouse carrying a human melanoma tumor indicated by a red line (left). On overlay of said image with the pyruvate (middle) and lactate signal (right) taken about 20 seconds after injection into the tail vein.
DOI: https://doi.org/10.58530/2023/4334