4330
SABRE Hyperpolarization of Bulky, Nitrile Containing Anti-Cancer Drugs, Letrozole and Anastrozole, with Long Hyperpolarization Lifetimes1Chemistry, North Carolina State University, Raleigh, NC, United States, 2Wayne State University, Detroit, MI, United States, 3Karmanos Cancer Institute, Detroit, MI, United States, 4Russian Academy of Science, Moscow, Russian Federation, 5University of North Carolina, Chapel Hill, NC, United States
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Cancer
Signal Amplification By Reversible Exchange in Shields Enable Alignment Transfer to Heteronuclei (SABRE-SHEATH) was employed to boost 15N magnetic resonance sensitivity on common anti-cancer agents. The SABRE-SHEATH hyperpolarization dynamics of letrozole and anastrozole were independently optimized with respect to solution temperature and polarization transfer field. These studies enabled single scan 15N detection of the drugs at natural isotopic abundance working with mM concentrations for over 30 minutes, paving the way for more sensitive drug development.
Introduction
Nuclear Magnetic Resonance (NMR) is a leading tool for characterizing ligand-protein interactions in drug screening.[1-3] The advantage with this non-destructive technique stems from the inherent chemical shift resolution afforded by NMR, which offers structural and functional information of ligand-protein interactions. However, NMR has inherently low sensitivity compared to other spectroscopic methods. This is because thermal spin polarization is microscopic (<10-5 at 3T for 15N) due to the small energy gap between Zeeman energy spin states, even at high magnetic fields and low temperatures. Hyperpolarization modalities increase the sensitivity of NMR by perturbing thermal spin polarization towards unity.Currently, the dominant hyperpolarization modality employed to characterize ligand-protein interactions is Dynamic Nuclear Polarization (DNP). However, in addition to being a lengthy process (~1hr), instrumentation for DNP is expensive (>1M), requiring cryogenic temperatures, a superconducting magnet, and high-powered microwave sources.[4] In contrast, Signal Amplification By Reversible Exchange (SABRE),[5] a ParaHydrogen Induced Polarization (PHIP) technique, can produce hyperpolarized (HP) substrates quickly (~1 minute or less) with little instrumentation cost (~$20k).
A number of FDA-approved drugs have been HP via SABRE to date.[6] The SABRE hyperpolarization scheme for a majority of the drugs referenced above is illustrated in Figure 1A. In this work, we utilize SABRE in Shield Enables Alignment Transfer to Heteronuclei (SABRE-SHEATH)[7] to extend the 15N heteronuclear scope to anti-cancer drugs, letrozole and anastrozole, shown in Figure 1B. Both drugs are on the World Health Organizations Model List of Essential Medicine, making them highly attractive biological compounds. This work not only broadens the scope of accessible substrates for SABRE-SHEATH, but also presents a methodical pathway for hyperpolarizing new SABRE substrates.
Methods
Specifically, we perform hyperpolarization build-up and lifetime studies and then optimize the hyperpolarization process with respect to solution temperature and the polarization transfer field (PTF). All samples are prepared using standard Schlenk line conditions. Samples are prepared with 30 mM substrate and 3 mM pre-catalyst (Ir-IMes) in 500 μL deuterated methanol. Samples are then transferred into a 7” medium wall NMR tube and subjected to bubbling under 100 psi of parahydrogen for 5 minutes prior to experimentation.Results/Discussion
To begin optimizing SABRE hyperpolarization for the drugs, we first conducted polarization build-up experiments on the nitrile substituents. Letrozole and anastrozole had similar build-up profiles, both reaching a steady state around 1 minute, with 15N Tb (build-up constant) of 21.7±1.4 seconds and 14.6±0.9 respectively. The experimental build-up data was fit using a mono-exponential function and are shown together in Figure 2A.Since studies using HP agents end with the decay of the HP signal, long lifetimes of HP states are not only convenient, but vital for monitoring biological (in vivo) and biochemical processes (in vitro: e.g., protein and drug-protein studies) that have slow or downstream interactions of interest. Agreeing with trends characterized in literature, short lifetimes are observed at low and high fields, while longer lifetimes are observed at intermediate fields.[8] Figure 2B shows the remarkably long 15N HP lifetime data recorded at 1 T, overlaid with mono-exponential fits yielding [15N]T1 of 554.1±12.7 s (>9 minutes) and 420.8±7.3 s (>7 minutes) for letrozole and anastrozole, respectively.
SABRE relies on the simultaneous association and dissociation of p-H2 and a target substrate on a transition metal catalyst. Recent advances in literature demonstrate the utility of modulating exchange by direct heating or cooling of the sample.[8] Following these findings, a temperature sweep was performed on both target drugs from room temperature (25 °C) to 50 °C with 5 °C increments, shown in Figure 2C. Prominently, we observed an increase in polarization as temperature was increased.
In traditional SABRE-SHEATH experiments, the sample is subjected to a very low (<1 μT) static field, where matching conditions are expected, aiding in polarization build-up. For this reason, we investigated the μT regime from 0 to 3 μT with 0.3 μT increments with static PTFs aligned parallel (+) and antiparallel (-) to the detection field, as shown in Figure 2D. Hyperpolarization was most efficiently generated at ±0.3 μT for both drugs.
Conclusion
We demonstrate the feasibility of hyperpolarizing new bulky anti-cancer drugs through SABRE. The feasibility of studying SABRE hyperpolarization at natural abundance of 15N and using benchtop NMR spectrometers substantially streamlines the experimental workflow, and potentially enables screening of many other nitrile-containing drugs, biomolecules, etc. This work lays a foundation that can readily be translated into highly sensitive screening of drug binding to protein targets using high-resolution NMR spectroscopy.Acknowledgements
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Nos. NIH R21EB025313 and NIH R01EB029829. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, we acknowledge funding from the Mallinckrodt Foundation, the National Science Foundation under Award No. NSF CHE-1904780, from the National Cancer Institute under Award No. NCI 1R21CA220137, and from the North Carolina Biotechnology Center in the form of a Translational Research Grant. Finally, we would like to acknowledge the support from NCSU’s METRIC providing access to NMR instrumentation.
References
[1] Chappuis, Q.; Milani, J.; Vuichoud, B.; Bornet, A.; Gossert, A. D.; Bodenhausen, G.; Jannin, S. Hyperpolarized Water to Study Protein-Ligand Interactions. J. Phys. Chem. Lett. 2015, 6 (9), 1674–1678. https://doi.org/10.1021/acs.jpclett.5b00403.
[2] Wang, Y.; Hilty, C. Amplification of Nuclear Overhauser Effect Signals by Hyperpolarization for Screening of Ligand Binding to Immobilized Target Proteins. Anal. Chem. 2020, 92 (20), 13718–13723. https://doi.org/10.1021/acs.analchem.0c01071.
[3] Kim, Y.; Hilty, C. Applications of Dissolution-DNP for NMR Screening. Methods Enzymol. 2019, 615, 501–526. https://doi.org/10.1016/BS.MIE.2018.08.016.
[4] Pinon, A. C.; Capozzi, A.; Ardenkjær-Larsen, J. H. Hyperpolarization via Dissolution Dynamic Nuclear Polarization: New Technological and Methodological Advances. Magnetic Resonance Materials in Physics, Biology and Medicine. 2021, pp 5–23. https://doi.org/10.1007/s10334-020-00894-w.
[5] Adams, R. W.; Duckett, S. B.; Green, R. A.; Williamson, D. C.; Green, G. G. R. A Theoretical Basis for Spontaneous Polarization Transfer in Non-Hydrogenative Parahydrogen-Induced Polarization. J. Chem. Phys. 2009, 131 (19). https://doi.org/10.1063/1.3254386.
[6] Barskiy, D. A.; Knecht, S.; Yurkovskaya, A. V.; Ivanov, K. L. SABRE: Chemical Kinetics and Spin Dynamics of the Formation of Hyperpolarization. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 114–115, 33–70. https://doi.org/10.1016/j.pnmrs.2019.05.005.
[7] Theis, T.; Truong, M. L.; Coffey, A. M.; Shchepin, R. V.; Waddell, K. W.; Shi, F.; Goodson, B. M.; Warren, W. S.; Chekmenev, E. Y. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 2015, 137 (4), 1404–1407. https://doi.org/10.1021/ja512242d.
[8] MacCulloch, K.; Tomhon, P.; Browning, A.; Akeroyd, E.; Lehmkuhl, S.; Chekmenev, E. Y.; Theis, T. Hyperpolarization of Common Antifungal Agents with SABRE. Magn. Reson. Chem. 2021, 59 (12), 1225–1235. https://doi.org/10.1002/mrc.5187.
Figures
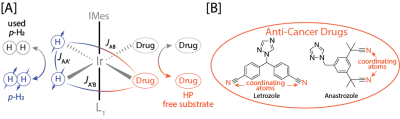
Figure 1. [A] Illustration of parahydrogen and a drug reversibly binding to a transition metal complex forming a temporarily J-coupling network, and [B] the anti-cancer drugs, letrozole and anastrozole, with target 15N nuclei (at natural isotopic abundance) highlighted in orange.
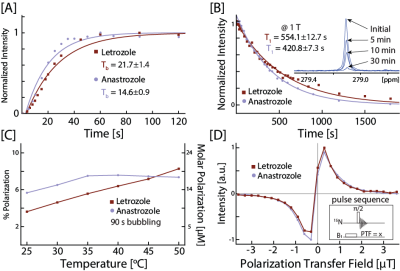
Figure 2. Optimization studies for the nitrile substituents nitrogen on letrozole and anastrozole. [A] Experimental polarization build-up data overlaid with mono-exponential fits. [B] Experimental [15N]T1 data recorded at 1 T overlaid with mono-exponential fits. [C] Temperature sweep from 25°C to 50°C with 5°C increments. [D] A static field sweep from 0 to 3.6 μT with 0.3 μT increments with PTFs both parallel (+) and antiparallel (-) to the detection field.