4320
Probing restriction and exchange in the human brain using free waveforms on a high-performance gradient system1Medical Radiation Physics, Lund, Lund University, Lund, Sweden, 2GE Research, Niskayuna, New York, NY, United States, 3Department of Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States, 4Clinical Sciences Lund, Lund University, Lund, Sweden
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Microstructure
Monitoring time dependence with diffusion MRI provides observables sensitive to restricted diffusion and exchange. Probing these phenomena by simply varying the diffusion time is a challenge because they have opposite effects on the diffusion-weighted signal and may cancel each other. A theoretical framework was recently proposed for disentangling the effects using free gradient waveforms. Here we explore the potential of the approach for neuroimaging in vivo using a high-performance gradient system. Results demonstrate unprecedented ability to disentangle the effects of restriction and exchange. Maps of the exchange rate show plausible contrast in both cortical and subcortical regions of the brain.Introduction
Water molecules diffusing in environments with permeable membranes exhibit time-dependent diffusion, which can be probed with diffusion MRI for mapping of observables related to both the restriction length (size) and exchange rate (permeability) of the microstructure1–3. Mapping these features in vivo is important for the characterisation of pathology and treatment response4–6. However, estimation of size and exchange by varying the diffusion time alone is a challenge because the two phenomena have opposite effects on the diffusion-weighted signal7,8. We recently proposed the use of free gradient waveforms to address this challenge9. Here, we utilise this novel approach to map restriction and exchange in vivo using a high-performance gradient system.Theory
The framework is based on a signal representation given by9$$\ln (S/S_0)\approx -b\left[ E_{\beta_0}+V_{\omega}E_{\beta_2}\right] + \frac{1}{2}b^2\left[V_{\beta_ 0}+2V_{\omega}C_{\beta_0\beta_2}+V_{\omega}^2V_{\beta_2}\right]\cdot (1-k{\Gamma}),\quad\quad (1)$$
where $$$ S_0$$$ is the non-diffusion-weighted signal and $$$b$$$ is the b-value ($$$b=\int_0^Tq(t)^2dt$$$), $$$q$$$ is the dephasing q-vector ($$$q(t)=\gamma \int _0^tg(\tau)d\tau$$$ where $$$g(\tau)$$$ is the gradient waveform), and $$$\gamma$$$ is the gyromagnetic ratio. Furthermore, $$$V_{\omega}$$$ is the restriction weighting
$$V_{\omega}=\frac{1}{2\pi b}\int_{-\infty}^{\infty}|q(\omega)|^2\omega ^2d\omega = \frac{\gamma^2}{b}\int_0^Tg^2(t)dt,\quad\quad (2)$$
where $$$q(\omega)$$$ is the Fourier transform of $$$q(t)$$$, and $$$\Gamma$$$ is the exchange weighting
$$\Gamma = 2\int_0^Tt\tilde{q_4}(t)dt,\quad\quad (3)$$
where $$$\tilde{q_4}(t)=q_4(t)/b^2$$$ and $$$q_4(t)=\int _0^Tq^2(t')q^2(t'+t)dt'$$$. The representation features three experimental parameters ($$$b,V_{\omega}$$$ and $$$\Gamma$$$) and six microstructure-related parameters: $$$E_{\beta_0}=\langle\beta_0\rangle$$$ is the mean time-independent diffusivity, $$$E_{\beta_2}=\langle\beta_2\rangle$$$ is the average restriction coefficient, $$$k$$$ is the exchange rate, $$$V_{\beta_0}$$$ and $$$V_{\beta_2}$$$ are the variances in $$$\beta_0$$$ and $$$\beta_2$$$, and $$$C_{\beta _0\beta_2}$$$ is their covariance. The principle is that independently varying $$$V_{\omega}$$$ and $$$\Gamma$$$ enables us to disentangle effects of restriction and exchange.
Methods
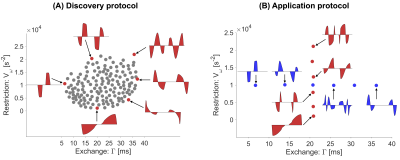
Two experiments were performed on a single volunteer using a 42-cm diameter head-only gradient – MAGNUS 10 at 3.0 T, capable of a maximum gradient strength of 200 mT/m and slew rate of 500 T/m/s. To span the $$$V_{\omega}$$$ vs $$$\Gamma$$$ space, the experiments featured multiple gradient waveforms, limited to a gradient strength of 200 mT/m, and a slew rate of 220 T/m/s to satisfy PNS limits. Two experiments were performed, both had the same pulse sequence parameters (TE=115 ms, TR=3 s, resolution = 2x2x5 mm3), whereas the diffusion encoding waveform sets were varied.The first experiment was a ‘discovery protocol’ tailored to explore the change in contrast from changing the exchange and restriction weighting. To this end, 150 unique waveforms with different values of $$$V_{\omega}$$$ and $$$\Gamma$$$ were generated (Fig.1A). The waveforms were applied along a single direction (y-direction) at three b-values (0, 2 and 4 ms/µm2). The scan time was 15 minutes.
The second experiment was the ‘application protocol’ and aimed at estimating restriction and exchange-related parameters. For this purpose, twelve waveforms were selected such that six had a constant $$$V_{\omega}$$$ but varying $$$\Gamma$$$ and six had fixed $$$\Gamma$$$ but varying $$$V_{\omega}$$$ (Fig.1B). Each waveform was applied in 10 directions per b-value using the b-values $$$0,1,2,3$$$ and $$$4$$$ ms/µm2. The scan time was 25 minutes.
Data were analysed by signal inspection in various regions of interest, by computing difference images between, for example, strong and weak exchange weighting at a fixed restriction weighting, and by fitting Eq.1 to powder-averaged signals using lsqnonlin in MATLAB (MathWorks, Natick, MA, USA; R2019a).
Results
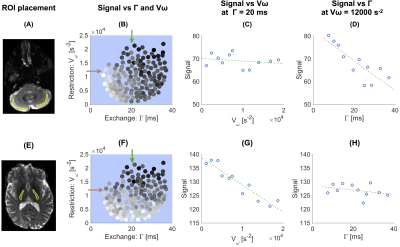
Fig.2 shows the signal at a fixed b-value for waveforms in the discovery protocol. The signal varies smoothly with $$$V_{\omega}$$$ and $$$\Gamma$$$, indicating that these parameters capture the salient features of the diffusion encoding waveforms. Effects of exchange dominate in the cerebellar cortex whereas restriction dominates in the internal capsule (white matter).
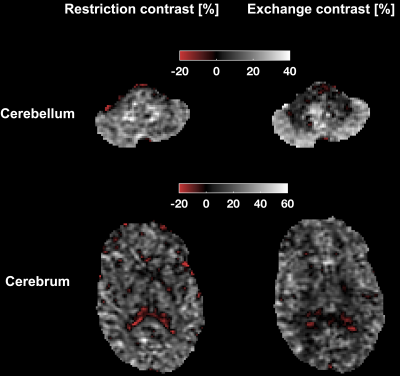
Fig.3 shows the restriction and exchange contrasts from the application protocol. The contrasts were obtained by subtracting images acquired with maximum and minimum $$$V_{\omega}$$$ and $$$\Gamma$$$ at b = 4 ms/µm2. Results again indicate that time dependence in grey matter is dominated by exchange, while restricted diffusion is dominant in white matter.
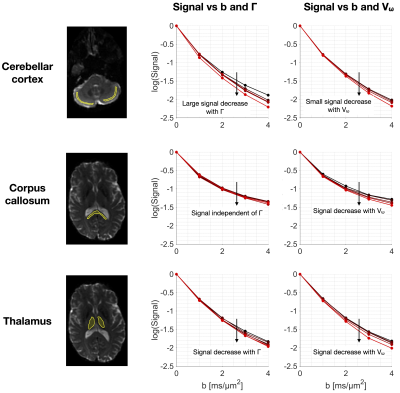
Fig.4 shows powder-averaged signal-vs-b curves from the application protocol in three brain regions. Time-dependence is driven by exchange in grey matter while restriction dominates in white matter. In the thalamus, both effects appear relevant.
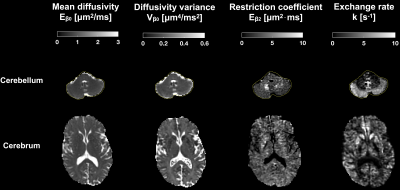
Parameter estimates obtained from fitting Eq.1 to the data from the application protocol are shown in Fig.5. There are high values of the restriction-related parameter $$$E_{\beta_2}$$$ in the cerebellar white matter and high exchange rates in the cerebellar cortex. Both results are in harmony with the contrasts in Fig.3.
Discussion
This work demonstrated that time dependence in vivo is well-captured by two experimental parameters: one for restriction weighting and another for exchange weighting. To the best of our knowledge, this is the first time that exchange in living tissue has been probed using this broad a set of waveforms with unique timing parameters. This was enabled by the availability of ultra-strong gradients.Parameter maps indicate slow exchange in white matter—suggesting that axons are effectively impermeable at these time scales11. Furthermore, exchange estimates in grey matter align with the upper end of the literature range (4 ms to over 100 ms8,12). Recent work suggests faster exchange in grey matter8, but we note that the framework used herein underestimates fast exchange rates9.
A limitation of this work is that ignores other effects such as structural disorder13 and intra-compartmental kurtosis14 which may be potential confounders15.
Acknowledgements
The authors acknowledge the funding support from Massachusetts Life Science Foundation, Swedish Cancer Society (22 0592 JIA), Swedish Research Council (2021-04844), NIH grants R01NS125781, P41EB015902References
1. Reynaud O. Time-Dependent Diffusion MRI in Cancer: Tissue Modeling and Applications. Front Phys. 2017;5. doi:10.3389/fphy.2017.00058
2. Kärger J. NMR self-diffusion studies in heterogeneous systems. Advances in Colloid and Interface Science. 1985;23:129-148. doi:10.1016/0001-8686(85)80018-X
3. Callaghan PT, Coy A, MacGowan D, Packer KJ, Zelaya FO. Diffraction-like effects in NMR diffusion studies of fluids in porous solids. Nature. 1991;351(6326):467-469. doi:10.1038/351467a0
4. Jiang X, Devan SP, Xie J, Gore JC, Xu J. Improving MR cell size imaging by inclusion of transcytolemmal water exchange. NMR in Biomedicine. n/a(n/a):e4799. doi:10.1002/nbm.4799
5. Volles MJ, Lee SJ, Rochet JC, et al. Vesicle Permeabilization by Protofibrillar α-Synuclein: Implications for the Pathogenesis and Treatment of Parkinson’s Disease. Biochemistry. 2001;40(26):7812-7819. doi:10.1021/bi0102398
6. Ruggiero MR, Baroni S, Pezzana S, Ferrante G, Geninatti Crich S, Aime S. Evidence for the Role of Intracellular Water Lifetime as a Tumour Biomarker Obtained by In Vivo Field-Cycling Relaxometry. Angewandte Chemie International Edition. 2018;57(25):7468-7472. doi:10.1002/anie.201713318
7. Nilsson M, Lätt J, Nordh E, Wirestam R, Ståhlberg F, Brockstedt S. On the effects of a varied diffusion time in vivo: is the diffusion in white matter restricted? Magnetic Resonance Imaging. 2009;27(2):176-187. doi:10.1016/j.mri.2008.06.003
8. Olesen JL, Østergaard L, Shemesh N, Jespersen SN. Diffusion time dependence, power-law scaling, and exchange in gray matter. NeuroImage. 2022;251:118976. doi:10.1016/j.neuroimage.2022.118976
9. Chakwizira A, Westin CF, Brabec J, et al. Diffusion MRI with pulsed and free gradient waveforms: Effects of restricted diffusion and exchange. NMR in Biomedicine. n/a(n/a):e4827. doi:10.1002/nbm.4827
10. Foo TKF, Tan ET, Vermilyea ME, et al. Highly efficient head-only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magn Reson Med. 2020;83(6):2356-2369. doi:10.1002/mrm.28087
11. Novikov DS, Fieremans E, Jespersen SN, Kiselev VG. Quantifying brain microstructure with diffusion MRI: Theory and parameter estimation. NMR in Biomedicine. 2019;32(4):e3998. doi:10.1002/nbm.3998
12. Jelescu IO, Palombo M, Bagnato F, Schilling KG. Challenges for biophysical modeling of microstructure. Journal of Neuroscience Methods. 2020;344:108861. doi:10.1016/j.jneumeth.2020.108861
13. Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proceedings of the National Academy of Sciences. 2014;111(14):5088-5093. doi:10.1073/pnas.1316944111
14. Henriques RN, Jespersen SN, Shemesh N. Evidence for microscopic kurtosis in neural tissue revealed by correlation tensor MRI. Magnetic Resonance in Medicine. 2021;86(6):3111-3130. doi:10.1002/mrm.28938
15. Lee HH, Papaioannou A, Novikov DS, Fieremans E. In vivo observation and biophysical interpretation of time-dependent diffusion in human cortical gray matter. Neuroimage. 2020;222:117054. doi:10.1016/j.neuroimage.2020.117054
Figures