4319
NODDI-based identification of white matter tracts in regions of peritumoral edema: a validation study in brains bearing meningioma tumors.1School of Biological and Health Systems Engineering, Arizona State University, Tempe, AZ, United States, 2Mayo Clinic, Scottsdale, AZ, United States, 3Vanderbilt University, Nashville, TN, United States
Synopsis
Keywords: White Matter, Diffusion/other diffusion imaging techniques
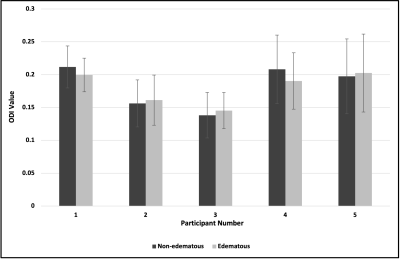
White matter (WM) tract detection proximal to brain tumors, surrounded by vasogenic edema during tumor resection is critical. Previous work has shown that NODDI presents a more accurate quantification of WM in edema as compared to DTI. However, these studies included gliomas and metastases, which are known to infiltrate WM. Here, we focus on validating the use of the NODDI model for WM identification in edema only in non-invasive meningiomas. Our results showed that DTI-derived FA of WM near edematous regions were lower than contralateral WM in non-edematous regions; in contrast, NODDI-derived ODI values for the same regions remained comparable.
Introduction
Accurate identification of white matter (WM) tracts that run proximal to brain tumors is of critical importance to the planning and surgical resection of the tumor. Misidentification of WM can result in unnecessary damage to a tract or undertreatment of the tumor. Standard-of-care diffusion tensor imaging (DTI) performs exceptionally well at identifying healthy WM tracts in a normal appearing brain, however, this method can fail to identify healthy WM in regions of tumor-associated vasogenic edema. This is because the presence of edema has the effect of increasing the fractional volume of isotropically diffusing water, thereby confounding the efforts of DTI to identify WM based on anisotropic diffusion alone.Recent work has shown that the Neurite Orientation Dispersion and Density Index (NODDI) model of diffusion in the central nervous system, when combined with multi-shell diffusion MRI, can better identify WM tracts in regions of peritumoral vasogenic edema relative to standard DTI1,2,3. In principle, this is because NODDI effectively isolates the rapidly and isotropically diffusing water (“free water”; in this case representative of edema) from restricted water that is presumed to be within neurites (dendrites and axons). However, an acknowledged limitation of this prior study was the fact that the vast majority of patients enrolled had invasive gliomas or metastases, which can compromise WM integrity.
A well-controlled study validating NODDI-based identification of WM tracts in regions of known pure edema would be a significant step towards widespread adoption. Here, we compare NODDI- and DTI-based identification of WM tracts through edema in human brains bearing non-invading meningioma – a tumor type that is well understood to generate significant vasogenic edema while retaining WM tract integrity.
Methods
Subject population: Adult subjects bearing radiologically-confirmed meningiomas were processed for this study (n = 5). Neuroimaging data acquisition: Imaging data were acquired on a 3T Siemens scanner using a 20-channel coil. Data were motion corrected using least squares minimization. Spatial smoothing of 2-4 mm3 was applied using the SPM12 toolbox4. The following scans were collected for clinical assessment: (i) a three-dimensional (3D) axial T1 magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence, (ii) a 3D post-Gadolinium (Gd) T1 MPRAGE sequence (TR/TE 1900/2.02 ms, 1 mm isotropic matrix, 256×256 mm FOV, 208 slices), (iii) a 3D T2 fluid-attenuated inversion recovery (FLAIR) sequence (TR/TE 5000/389 ms, 192 slices, and (iv) multi-shell diffusion weighted acquisitions with the following parameters: b-values= 700, 1000, 1500, 1900, 2000 & 2500 s/mm2 (TR/TE 3664/95.6 ms, 185 slices); flip-angle = 90°; acquisition matrix: 104 × 104; slice thickness: 2.5 mm; number of averages = 1 and one b= 0 s/mm2 image at the beginning of the acquisition. All MR images were screened for neuropathology by a licensed neuroradiologist prior analysis. Diffusion neuroimaging preprocessing: Data were noise corrected5 and a synthetic b06 algorithm was applied to generate reverse phase-encoded images before correcting for motion distortion and eddy currents7. DTI8 and NODDI9,10 models were applied to preprocessed data to yield fractional anisotropy (FA) and orientation dispersion index (ODI) maps respectively. Diffusion neuroimaging postprocessing: The tumor was identified and segmented in a T1-w post contrast image. The tumor contour thus obtained was then overlaid on a T2-FLAIR, followed by segmentation of the hyperintense signal representing edema around the tumor in the T2-FLAIR. The region highlighting extra-tumoral edema was derived after subtracting the two segmentations (i.e., T2 FLAIR region of interest – T1 post contrast region of interest = (edema + tumor) – tumor). In each patient, regions of interest (ROIs) were drawn (on the ODI map) in the white matter tract proximal to edematous tissue as well as the corresponding white matter tract of the contralateral hemisphere; the location of each ROI was confirmed by a licensed neuroradiologist. All images were co-registered to a T1-w image. Statistical analysis: A Shapiro-Wilks test assessed the FA and ODI distributions for normality and a t-test determined if the ODI values for the edematous and non-edematous white matter ROIs were significantly different.Results
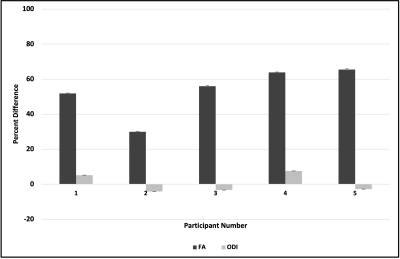
Our results indicated that FA of white matter tracts near edematous regions were systematically lower than contralateral white matter tracts in non-edematous regions (Fig. 1); in contrast, ODI values for these same white matter tracts remained comparable (Fig. 2). Figure 3 illustrates the percentage difference between FA and ODI values for each white matter region of interest in edematous and non-edematous regions (see Eqs. 1 and 2). Overall, the FA of white matter tracts in edematous and non-edematous regions yielded disparate values, whereas the ODI of these same white matter tracts remained comparable despite the presence of edema.Eq.1: ($$$\frac{FA_{edematous}- FA_{non-edematous}}{FA_{edematous}}\times100$$$)
Eq.2: ($$$\frac{ODI_{edematous}- ODI_{non-edematous}}{ODI_{edematous}}\times100$$$)
Discussion and Conclusion
In this validation study, we compared the mean FA and ODI values between regions of interest in white matter tracts in edematous and non-edematous regions of brains bearing meningiomas. Results indicated that ODI values within white matter tracts in edema were comparable to those in non-edematous regions, suggesting that ODI could provide clinical utility in localizing critical white matter tracts prior tumoral resection. Future work will aim to histologically validate this approach.Acknowledgements
No acknowledgement found.References
- Yen, P. S., Teo, B. T., Chiu, C. H., Chen, S. C., Chiu, T. L., & Su, C. F. (2009). White matter tract involvement in brain tumors: a diffusion tensor imaging analysis. Surgical Neurology, 72(5), 464-469.
- Masjoodi S, Hashemi H, Oghabian MA, Sharifi G. Differentiation of Edematous, Tumoral and Normal Areas of Brain Using Diffusion Tensor and Neurite Orientation Dispersion and Density Imaging. J Biomed Phys Eng. 2018 Sep 1;8(3):251-260. PMID: 30320029; PMCID: PMC6169116.
- Chong, S. T., Liu, X., Kao, H. W., Lin, C. Y. E., Hsu, C. C. H., Kung, Y. C., ... & Lin, C. P. (2021). Exploring peritumoral neural tracts by using Neurite Orientation Dispersion and Density Imaging. Frontiers in neuroscience, 15, 702353.
- fil.ion.ucl.ac.uk/spm/software/spm12/
- mrtrix.readthedocs.io/en/dev/dwi_preprocessing/denoising.html
- Schilling, K. G., Blaber, J., Huo, Y., Newton, A., Hansen, C., Nath, V., ... & Landman, B. A. (2019). Synthesized b0 for diffusion distortion correction (Synb0-DisCo). Magnetic resonance imaging, 64, 62-70.
- FMRIB, Oxford, UK [Andersson 2016a] Jesper L. R. Andersson and Stamatios N. Sotiropoulos. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125:1063-1078, 2016.
- fsl.fmrib.ox.ac.uk/fslcourse/2019_Beijing/lectures/FDT/fdt1.html
- mig.cs.ucl.ac.uk/index.php?n=Tutorial.NODDImatlab
Zhang, H., Schneider, T., Wheeler-Kingshott, C. A., & Alexander, D. C. (2012). NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage, 61(4), 1000-1016
Figures
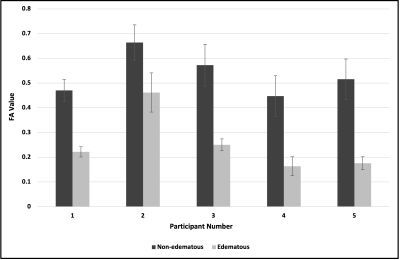
Figure 1: Comparison of FA values between white matter regions of interest in edematous and non-edematous regions.