4300
Single-shot, Multi-TI Late Gadolinium Enhancement MRI using GRASP-Pro Reconstruction with View-Sharing and KWIC Filtering1Biomedical Engineering, Northwestern University, Evanston, IL, United States, 2Radiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 3BioMedical Engineering and Imaging Institute(BMEII), Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Division of Cardiology, Internal Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
Synopsis
Keywords: Myocardium, Tissue Characterization
Late gadolinium enhancement (LGE) is the clinical standard for assessment of myocardial scarring. Current limitations of standard LGE are lengthy scan time, sensitivity to arrhythmia and/or dyspnea, and reliance of optimal inversion time (TI), which may be difficult to identify for patients with subendocardial scarring. We propose a free-breathing, single-short, multi-TI LGE approach to address the aforementioned challenges. Our results show that GRASP-Pro reconstruction with view-sharing (VS) and k-space weighted image contrast (KWIC) filtering produces multi-TI LGE images (i.e., no need for TI scout) with relatively high spatial resolution.Introduction
Late gadolinium enhancement (LGE) is the gold standard for evaluation of myocardial scarring1. Limitations of breath-hold segmented LGE include long scan time and sensitivity to arrhythmia and/or dyspnea; limitation of single-shot LGE include low spatial resolution; both LGE acquisitions rely on optimal inversion time (TI) selection, which requires expertise. We had previously developed a highly-accelerated multi-TI, single-shot LGE with radial k-space sampling and GRASP reconstruction2; its advantages are that it is a single-shot (two heartbeats), produces multi-TI images, which eliminates need for a TI scout. While promising, the initial images produced relatively blurry images resulting from high acceleration factor (16-fold). The objective of this study was to incorporate view sharing (VS) and k-space weighted image contrast (KWIC) filtering3 into GRASP-Pro4 to achieve 25-fold acceleration and demonstrate the benefits of VS and KWIC for reducing image blurring.Methods
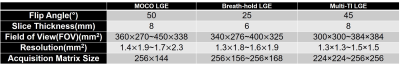
Human subjects and pulse sequence:We scanned 21 patients (mean age= 60.0±16.5 years; 13 males; 8 females) approximately 10-15 min following administration of 0.15 mmol/kg of gadobutrol at 1.5 Tesla (Siemens; Aera and Avanto); 11 patients were scanned with clinical breath-holding (BH) LGE and multi-TI, single-shot LGE; 10 patients were scanned with clinical motion-corrected free-breathing (MOCO) LGE5 and Multi-TI, single-shot LGE. Relevant patient demographics and imaging parameters are summarized in Table 1 and Table 2, respectively.
Image reconstruction and quantitative analysis:
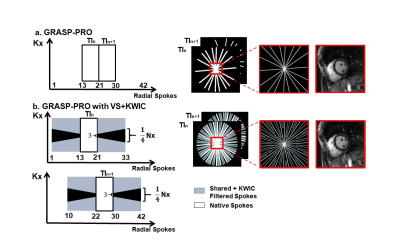
For the conventional GRASP-Pro method, we reconstructed 20 TI frames using 9 radial spokes per frame (acceleration factor 25). For GRASP-Pro with VS+KWIC, we used VS of 33 radial spokes per frame, where 12 radial spokes immediately before and after the 9 native spokes were shared from adjacent frames. We then applied KWIC filter to zero out the central portion of shared k-space lines, which is necessary to reduce motion blurring and mixture of different TI inherent to radial k-space sampling. For illustration of sampling schemes, see Figure 1. Following GRASP-Pro and GRASP-Pro with VS+KWIC, we also applied temporal low-rank block wise filter to remove residual noise.
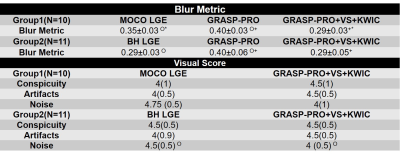
For quantitative analysis, the blur metric (0=sharp; 1=blur)6 was calculated for conventional GRASP-Pro LGE, GRASP-Pro LGE with VS+KWIC, clinical MOCO LGE, and clinical BH LGE for basal, mid, and apex slices. For the visual score, several TI frames before and after the best myocardial nulling point (optimal TI) of GRASP-Pro with VS+KWIC reconstructed Multi-TI LGE were examined to leverage multi-TI. The visual scores were graded on a 5-point Likert scale (1: worst; 3: clinically acceptable; 5: best) for each of three categories (conspicuity of myocardial scar or myocardium; artifact; noise), independently by one radiologist and one cardiologist. The variable normality was tested using the Shapiro-Wilk test. The blur metrics were compared using ANOVA with Bonferroni correction for pairwise comparison. The visual scores were compared using the Wilcoxon signed-rank test (if normally distributed, used paired t test).
Results
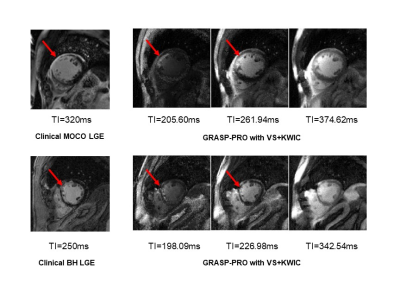
According to the Shapiro-Wilk test, all the variables were normally distributed except visual scores. The blur metric (see Table 3) was significantly (P<0.05) lower for GRASP-Pro LGE with VS+KWIC (0.29±0.03) than conventional GRASP-Pro LGE (0.40±0.03); hence, we evaluated VS+KWIC only for visual comparison with clinical standard LGE. For completeness, the blur metric was significantly lower (i.e., sharper) for GRASP-Pro LGE with VS+KWIC (0.29±0.03) than clinical MOCO LGE (0.35±0.03), but it was not significantly different compared with clinical BH LGE (0.29±0.03).Figure 2 shows representative clinical standard LGE (MOCO and BH LGE) and GRASP-Pro LGE with VS and KWIC of two different patients with myocardial scarring. Several frames before and after the optimal TI of the Multi-TI LGE were shown to provide temporal information of TI.
For visual scores summarizing all patients (see Table 3), the conspicuity and artifacts scores were not significantly different between BH LGE and GRASP-Pro LGE with VS+KWIC and between MOCO LGE and GRASP-Pro LGE with VS+KWIC. The noise level was significantly higher for GRASP-Pro LGE with VS+KWIC than clinical BH LGE (P<0.05). All visual scores were better than clinical acceptable (above 3.0).
Conclusion
This study suggests that incorporation of VS and KWIC filtering into GRASP-Pro reduces blurring – hence improves spatial resolution - for multi-TI, single-shot LGE obtained with radial k-space sampling and free-breathing. Future studies include quantification of myocardial scar volume and T1 calculation from multi-TI.Acknowledgements
National Institutes of Health (R01HL116895, R21AG055954, R01HL151079, R21EB030806A1,R01EB030549) and American Heart Association (19IPLOI34760317, 949899)References
1. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100(19):1992-2002.
2. Shen D, Hong K, Allen BD, Lee DC, Kim D. 16-fold accelerated, single-shot late gadolinium enhancement CMR using GRASP for multi-TI reconstruction. In: Proceedings of the 28th Annual Meeting of ISMRM. Virtual.
3. Song HK, Dougherty L. k space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med 2000;44(6):825-832.
4. Feng L, Wen Q, Huang C,Tong A , Liu F, Chandarana H. GRASP Pro: imProving GRASP DCE MRI through self calibrating subspace modeling and contrast phase automation.Magn Reson Med 2020; 83(1):94-108.
5. Kellman P, Larson AC, Hsu LY, Chung YC, Simonetti OP, McVeigh ER, Arai AE. Motion-corrected free-breathing delayed enhancement imaging of myocardial infarction. Magn Reson Med 2005;53(1):194-200.
6. Crete F, Dolmiere T, Ladret P, Nicolas M. The blur effect: perception and estimation with a new no-reference perceptual blur metric:SPIE;2007.
Figures