4297
Global, segmental and layer specific analysis of myocardial involvement in Duchenne muscular dystrophy by LGE1Shenzhen Children’s Hospital, shenzhen, China, 2Shantou University Medical College, shantou, China, 3Philips Healthcare, guangzhou, China
Synopsis
Keywords: Myocardium, Tissue Characterization
To provide more imaging basis for early identification and prognosis evaluation of DMD-associated cardiomyopathy(DMD-CM), we analyzed the distribution characteristics of LGE in global and regional left ventricle and the correlation between myocardial fibrosis index and cardiac function indexes in 29 boys with DMD. The range of LGE and the distribution pattern of interventricular septal involvement are related to the decrease of cardiac function. LGE is a reliable tool to identify subclinical DMD-CM and evaluate the severity of the disease.Introduction
Progressive cardiomyopathy is the main cause of death in patients with Duchenne muscular dystrophy (DMD). Early identification of heart involvement and active treatment are very important to maximize the duration of life and improve the quality of life1,2. LGE can not only accurately identify myocardial fibrosis, but also make risk stratification and prognosis assessment of the disease3,4. However, the average age in previous studies were old and they usually focused on the enhancement range of the whole myocardium, while ignoring the distribution heterogeneity of segments and layers. In this study, we aimed to analyze the distribution of LGE in global and regional left ventricle in young patients with DMD, and to explore the relationship between LGE and cardiac function indexes, so as to provide more imaging basis for early identification and prognosis evaluation of DMD-CM.Methods
29 boys with DMD (mean age 10.9 ±2.0years) were prospectively enrolled. All subjects underwent CINE and LGE sequence scanning on a 3.0T MR scanner. CVI software was used for post-processing. According to the American Heart Association (AHA)17-segment model, the endocardial and epicardial boundaries were drawn on the LGE images of the basal, middle and apical segments. The types of LGE were divided into extensive type (≥ 3 segments continuously involved), scattered type (< 3 segments intermittently involved) and mixed type5. The layer characteristics of each LGE positive segment were further analyzed and the myocardial fibrosis index ((sum of positive segments / total segments) × 100%) was calculated. Cardiac function indexes including EDVI, ESVI, SVI, CI and EF were measured on the short axis of CINE sequence. The mean between the two groups was tested by t-test. The correlation between myocardial fibrosis index and cardiac function index was analyzed by Pearson correlation analysis. All tests were bilateral tests, and it was considered that there was statistical difference when p < 0.05.Results
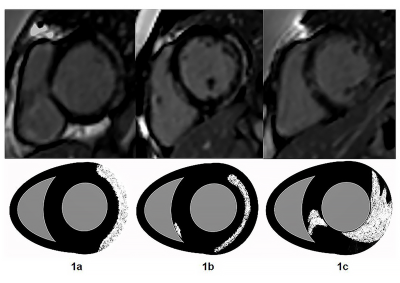
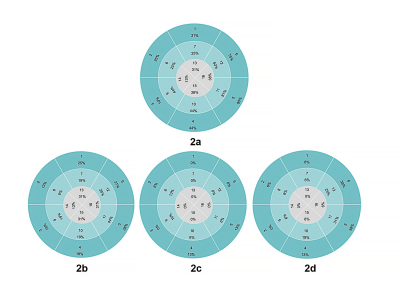
Of the 29 DMD patients, 16 (42%) were LGE positive and 13 (58%) were LGE negative. In the segmental analysis, 107 (42%) segments were positive in 16 LGE positive patients. The distribution of LGE was characterized by extensive involvement of subepicardial myocardium in the free wall (Figure 1a-c), and the most frequently involved segments were basal inferolateral, mid inferolateral and basal anterolateral segments (Figure 2a-d). There was no significant difference among basal segment, middle segment and apical segment. The LVEF (52.5 ±9.2% vs 58.4 ±2.6%, p=0.03) and CI ((3.4±0.8 vs 4.2±0.5L/(min×m2), p<0.01) of LGE positive patients were lower than those of LGE negative patients. The LVEF of patients with interventricular septal involvement was lower than that of patients with only free wall involvement (58.9±6.7% vs 47.6±5.8%,p=0.01). Left ventricular myocardial fibrosis index was negatively correlated with LVEF (r=-0.59,p=0.02) while positively correlated with EDVI (r = 0.80, p< 0.01) and ESVI (r = 0.77, p< 0.01). (Table1)Discussion
The role of LGE in predicting the occurrence and outcome of adverse cardiac events has been widely confirmed6,7. However, previous studies usually focus on the range of LGE and ignore the distribution pattern. Our study comprehensively analyzed the five characteristics of LGE distribution in the whole and regional left ventricle of patients with DMD, and further confirmed that the LGE of DMD-CM has a specific distribution pattern. The LVEF of patients with interventricular septum involvement was lower than that of patients with only free wall involvement, indicating that cardiac function was more seriously impaired when interventricular septum was involved, and this view was indirectly confirmed by MenonSC8 and HorKN9 et al. The results of Halliday10 et al on dilated cardiomyopathy showed that interventricular septum involvement was significantly associated with a significantly increased risk of sudden cardiac death (SCD). It is suggested that we should not only evaluate the existence and scope, but also pay attention to the distribution and pattern of LGE. In addition, our study showed that 35% of DMD patients with LVEF ≥ 55% were LGE positive. This suggests that myocardial fibrosis in DMD-CM exists before left ventricular global cardiac dysfunction. The purpose of using CMR in DMD patients has shifted from rescue treatment when abnormal LVEF is found to early treatment of occult cardiomyopathy with LGE positive but LVEF ≥ 55%11. Finally, studies on young DMD groups can help confirm the baseline age of CMR. Recent nursing guidelines12 recommend that young patients be examined by electrocardiogram, echocardiography (< 6-7 years old) and cardiac magnetic resonance imaging (≥ 6-7 years old) to assess myocardial involvement. In our study, the youngest patient was 7 years old, and the earliest LGE positive time was 9 years old. 16 cases (55%) of DMD patients had abnormal ECG results. Only 1 patient (3%) showed segmental wall motion abnormality by echocardiography. Cardiac magnetic resonance imaging (CMR) can provide accurate information about cardiac histology and function, which is of great significance for early identification and timely treatment of DMD-CM.Conclusion
The distribution of LGE in DMD was characterized by extensive involvement of subepicardial myocardium in the free wall. The range of LGE and the distribution pattern of interventricular septum involvement are related to the decline of cardiac function. LGE is a reliable tool to identify subclinical DMD-CM and evaluate the severity of the disease.Acknowledgements
Not applicable.References
1.Raman SV, Hor KN, Mazur W, et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: results of a two-year open-label extension trial. Orphanet journal of rare diseases 2017; 12(1): 39.
2. Russo V, Papa AA, Williams EA, et al. ACE inhibition to slow progression of myocardial fibrosis in muscular dystrophies. Trends in cardiovascular medicine 2018; 28(5): 330-7.
3.Tandon A, Villa CR, Hor KN, et al. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in duchenne muscular dystrophy. Journal of the American Heart Association 2015;4(4).
4. Menon SC, Etheridge SP, Liesemer KN, et al. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatric cardiology 2014; 35(7): 1279-85.
5.Wei X, Zhao L, Xie J, et al. Cardiac Phenotype Characterization at MRI in Patients with Danon Disease: A Retrospective Multicenter Case Series. Radiology 2021; 299(2): 303-10.
6. Halliday BP, Gulati A, Ali A, et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients With Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation2017; 135(22): 2106-15.
7. Disertori M, Rigoni M, Pace N, et al. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovascular imaging2016; 9(9): 1046-55.
8.Menon SC, Etheridge SP, Liesemer KN, et al. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatriccardiology2014; 35(7): 1279-85
9. Hor KN, Taylor MD, Al-Khalidi HR, et al. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. JACC Cardiovascular imaging2013; 15(1): 107.
10. Halliday BP, Baksi AJ, Gulati A, et al. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovascularimaging 2019; 12(8 Pt 2): 1645-55.
11.Lee S, Lee M, Hor KN. The role of imaging in characterizing the cardiac natural history of Duchenne muscular dystrophy. Pediatric pulmonology 2021; 56(4): 766-81.
12. Birnkrant
DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy,
part 2: respiratory, cardiac, bone health, and orthopaedic management. The
Lancet Neurology2018; 17(4): 347-61.
Figures