4296
Left Ventricular Vertical Run-Length Non-Uniformity Adds Prognostic Value to MACE in Patients with ESRD1Nephrology, Shanghai Jiaotong University School of Medicine Affiliated Renji Hospital, Shanghai, China, 2Philips Healthcare, Shanghai, China, Shanghai, China, 3Radiology, Shanghai Jiaotong University School of Medicine Affiliated Renji Hospital, Shanghai, China
Synopsis
Keywords: Myocardium, Tissue Characterization, End-stage renal disease
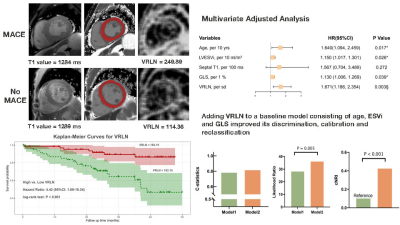
Left ventricular vertical run-length non-uniformity (VRLN) represents heterogeneity within native T1 images and reflects the extent of cardiac fibrosis. In uremic cardiomyopathy, interstitial fibrosis was found to be the major histological alteration. The aim of this study was to evaluate the prognostic value of VRLN in patients with ESRD. The primary endpoint was major adverse cardiac events (MACE). Survival analysis was calculated by Kaplan-Meier curves with log-rank test. VRLN remained associated with MACE in the multivariable model. Adding VRLN to a model containing clinical and CMR parameters significantly improved the accuracy and reclassification of the predictive model.Introduction
Major adverse cardiac events (MACE) significantly reduce the quality of life and are the leading cause of death in patients with ESRD1. The incidence of MACE is usually accompanied by alterations in myocardial morphology, function, and histology2,3,4. Diffuse interstitial fibrosis is the predominant myocardial histological feature of uremic cardiomyopathy5. Previous studies have proven that diffuse interstitial fibrosis is highly related to the incidence of MACE in several diseases6,7,8. Native T1 mapping has been applied to a number of cardiac conditions that lead to diffuse myocardial fibrosis, and most studies found a significant increase of myocardial T1 values compared to control subjects9,10,11,12. However, the overlap of T1 values between patients with ESRD and controls remains extensive, so the discrimination of diffuse myocardial fibrosis provided by native T1 in individual patients may be limited13.Texture analysis (TA) refers to the measurement of changes in pixel intensity on images, regions of interest (ROIs), or volume, which has recently been used as a method for exploring data contained in images too difficult to be explored visually14. Among 279 texture features, VRLN was significantly higher in patients on maintenance dialysis and had high sensitivity and accuracy in detecting interstitial fibrosis13. Therefore, based on the above studies, we assume that VRLN can be used as a novel predictor for MACE in patients with ESRD.Methods
Consecutive patients on maintenance dialysis due to ESRD and had undergone cardiac magnetic resonance (CMR) were prospectively enrolled from June 2018 to November 2020 in Renji Hospital. Patients were scanned using 3.0 T scanners (Ingenia, Philips, Best, The Netherlands). 279 texture features in 6 categories including VRLN of 127 participants were extracted from native T1 mappings by free software (MaZda version 4.6). Univariate hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression. Kaplan-Meier survival curves were estimated with VRLN and global T1 values as dichotomous parameters, based on the cubic spline cutoff value or median value, and differences in survival distributions were assessed by using the log-rank test.Results
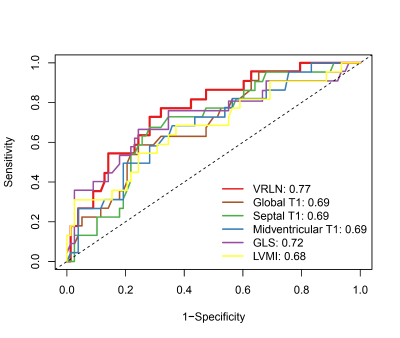
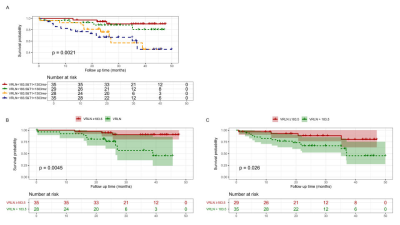
VRLN was significantly higher in patients with MACE compared to patients without MACE (223.47±60.38 vs. 173.11±56.32, P < 0.001). A total of 30 patients reached the primary outcome. ROC curves of VRLN, T1 mapping, LVMI, and GLS revealed that VRLN had the highest predictive accuracy of MACE. Kaplan-Meier survival curves demonstrated that the incidence of MACE, was higher in patients with high (≥ 183.15) VRLN compared to those with low (< 183.15) VRLN. After backward stepwise selection, age, ESVi, septal T1, GLS, and VRLN (HR: 1.671 [95% CI: 1.186-2.354; P = 0.003]) were entered into the multivariate regression analysis. The addition of VRLN improved the discrimination (C-statistic: 0.781 [95% CI: 0.708-0.854] vs. 0.814 [95% CI: 0.745-0.883]), goodness-of-fit (P = 0.005) and reclassification (cNRI: 0.424; P < 0.001) of the predictive model. In both subgroups of global T1 below or above median, patients with VRLN ≥ 183.15 had a significantly worse prognosis compared with patients with VRLN < 183.15 (T1 below median: P = 0.004; T1 above median: P = 0.026). In patients with global T1 below median, addition of VRLN to the baseline model consisting of age, ESVi, and GLS also improved the discrimination, goodness-of-fit, and reclassification.Discussion
In this current study, we found that VRLN was an independent predictor for MACE in patients on maintenance dialysis, and it was also associated with single outcome events including AMI or stroke, HF hospitalization, and life-threatening arrhythmia. Adding VRLN to a baseline model consisting of age, LV ESVi, and GLS significantly improved discrimination and reclassification ability of the predicting model for MACE. In the subgroup analysis, we evaluated the prognostic value of VRLN to MACE in participants with relatively low global T1 values (<1303 ms). VRLN remained independently associated with outcome and improved the discrimination and reclassification ability of the predictive model in this particular population. These results showed that when T1 mapping had not yet effectively identified myocardial alterations, the elevation in VRLN could predict MACE in patients with ESRD. Thus, including VRLN in ESRD patient management should be considered for improved risk stratification.Conclusion
In patients on maintenance dialysis due to ESRD, LV VRLN by CMR provides incremental prognostic value for MACE, independently of T1 mapping, ESVi, or GLS. Adding VRLN to baseline predictive model consisting of clinical and CMR conventional parameters significantly improved the discrimination and reclassification ability for MACE. Further studies are required to validate universality of the prognostic value and find ways to make VRLN widely available for clinical practice.Acknowledgements
This study was funded by National Natural Science Foundation of China, National Natural Science Foundation of China Youth project, Shanghai Science and technology innovation action plan, technology standard project and Shanghai Science and technology innovation action plan. The study was also sponsored by Shanghai Municipal Health Commission, Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant;Shanghai Jiao Tong University school of medicine Double hundred outstanding person project, Shanghai Jiao Tong University School of Medicine, Shanghai Outstanding Academic Leaders Plan as well as innovative research team of high-level local universities in Shanghai.References
1. Yang J-Y., Huang J-W., Chen L., et al. Frequency of Early Predialysis Nephrology Care and Postdialysis Cardiovascular Events. American Journal of Kidney Diseases 2017;70(2):164–72.
2. Lee H-J., Lee H., Kim SM., et al. Diffuse Myocardial Fibrosis and Diastolic Function in Aortic Stenosis. JACC: Cardiovascular Imaging 2020;13(12):2561–72.
3. Tomura M., Hamasaki Y., Komaru Y., et al. Prognostic significance of concentric left ventricular hypertrophy at peritoneal dialysis initiation. BMC Nephrol 2021;22(1):135.
4. Huang J-C., Su H-M., Wu P-Y., et al. Ratio of Early Mitral Inflow Velocity to the Global Diastolic Strain Rate and Global Left Ventricular Longitudinal Systolic Strain Predict Overall Mortality and Major Adverse Cardiovascular Events in Hemodialysis Patients. Disease Markers 2019;2019:1–12.
5. Wang X., Shapiro JI. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol 2019;15(3):159–75.
6. Li Y., Liu X., Yang F., et al. Prognostic value of myocardial extracellular volume fraction evaluation based on cardiac magnetic resonance T1 mapping with T1 long and short in hypertrophic cardiomyopathy. Eur Radiol 2021;31(7):4557–67.
7. Roy C., Slimani A., de Meester C., et al. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson 2018;20(1):55.
8. Pan JA., Kerwin MJ., Salerno M. Native T1 Mapping, Extracellular Volume Mapping, and Late Gadolinium Enhancement in Cardiac Amyloidosis. JACC: Cardiovascular Imaging 2020;13(6):1299–310.
9. Sado DM., White SK., Piechnik SK., et al. Identification and Assessment of Anderson-Fabry Disease by Cardiovascular Magnetic Resonance Noncontrast Myocardial T1 Mapping. Circ: Cardiovascular Imaging 2013;6(3):392–8.
10. Bull S., White SK., Piechnik SK., et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013;99(13):932–7.
11. Sparrow P., Messroghli DR., Reid S., Ridgway JP., Bainbridge G., Sivananthan MU. Myocardial T1 Mapping for Detection of Left Ventricular Myocardial Fibrosis in Chronic Aortic Regurgitation: Pilot Study. American Journal of Roentgenology 2006;187(6):W630–5.
12. Dass S., Suttie JJ., Piechnik SK., et al. Myocardial Tissue Characterization Using Magnetic Resonance Noncontrast T1 Mapping in Hypertrophic and Dilated Cardiomyopathy. Circ: Cardiovascular Imaging 2012;5(6):726–33.
13. Zhou H., An D., Ni Z., et al. Texture Analysis of Native T1 Images as a Novel Method for Noninvasive Assessment of Uremic Cardiomyopathy. J Magn Reson Imaging 2021;54(1):290–300.
14. Corrias G., Micheletti G., Barberini L., Suri JS., Saba L. Texture analysis imaging “what a clinical radiologist needs to know.” European Journal of Radiology 2022;146:110055.
Figures