4294
Low-dose Dobutamine Stress CMR perfusion for the Evaluation of Myocardial Microcirculation Induced by Chronic High-Altitude Hypoxia in Rats1Radiological department, Sichuan Academy of Medical Sciences – Sichuan Provincial People's Hospital(SAMSPH), Chengdu, China
Synopsis
Keywords: Heart, Perfusion
This study investigated the cardiovascular effects of altitude exposure, and determined the feasibility of stress first-pass perfusion CMR to objectively and noninvasively diagnose chronic HAH-induced microvascular changes. The results showed a significant decreased in LVEF as compared with NC animals at two time points. Our study has shown rest_RU and stress_RU were significantly lower and rest_MPI, stress_MPI and MPR were significantly higher. The pilot testing demonstrated that the feasibility of stress first-pass perfusion CMR to objectively and noninvasively diagnose chronic HAH-induced microvascular changes.Background:
During sustained exposure to high-altitude hypoxia (HAH) is known to induce multiple additional physiologic changes such as increase in heart rate, cardiac contractility and cardiac output that impact the cardiovascular system[1,2]. it is of great significance to develop effective methods to detect myocardial blood flow and function and reduce the risk of chronic HAH-associated cardiorespiratory diseases for native and long-term residents at high altitudes in the early stages[3,4]. This study aimed to investigate the cardiovascular effects of altitude exposure, and determined the feasibility of stress first-pass perfusion CMR to objectively and noninvasively diagnose chronic HAH-induced microvascular changes.Methods:
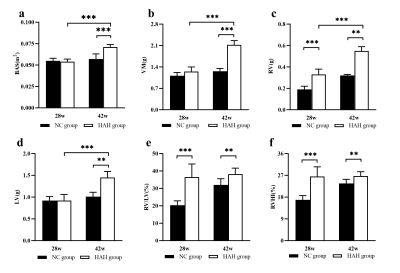
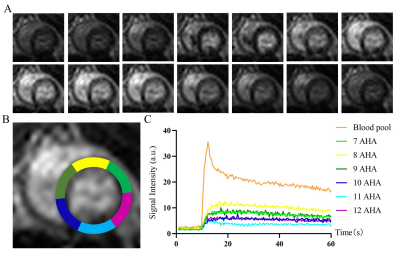
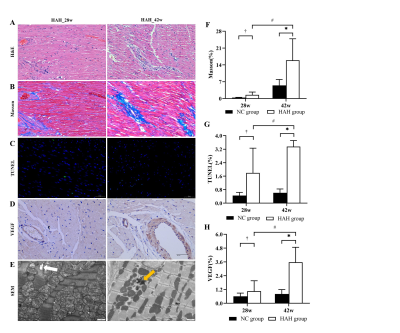
Methods: A total of 52 male SD rats were divided into in two groups and raised in different environments from 6 weeks of age for a period of 28 weeks and 42 weeks respectively. A 7.0T small animal magnetic resonance scanner was used to perform CMR scanning on rats. CVI 42 software was used to analyze the cardiac function and myocardial first-pass perfusion parameters including the left and right ventricular myocardial mass (LVM), left and right ventricular end-systolic volume (ESV), left and right ventricular end-diastolic volume (EDV), EF, RU rest, RU dobutamine, MPI rest, MPI dobutamine and MPRI were calculated using semiautomated endocardial and epicardial contour tracing based on a short-axis cine stack. Prior to the CMR scan, blood was collected from the two groups of rats for evaluation of blood indicators. After the scan, the rats were sacrificed and the myocardial tissue morphology, ultrastructure of myocardial cells, TUNEL apoptosis and VEGF immunohistochemical were detected.Results:
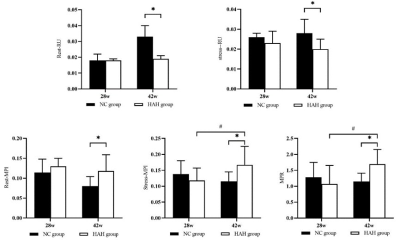
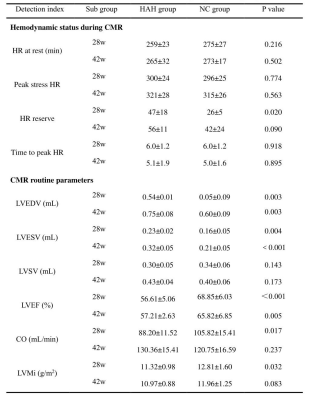
the HAH group in 42 weeks had significantly higher red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT) than the NC group in 42 weeks (p < 0.001). HAH rats displayed impaired cardiac function as demonstrated by decreased LVEF as compared with NC animals at two time points (LVEF in 28 weeks, 56.61±5.06% vs 68.85±6.03%, P < 0.01; LVEF in 42 weeks, 57.21±2.63% vs 65.82±6.85%, P < 0.01, respectively). it was found that rest_RU and stress_RU of HAH_42w group were significantly lower than those of NC_42w (0.019±0.002 vs 0.033±0.007 and 0.020±0.005 vs 0.028±0.007), and rest_MPI, stress_MPI and MPR in HAH_42w group were significantly higher than NC_42w (0.177±0.041 vs 0.080±0.024, 0.167±0.058 vs 0.115±0.57030 and 115±0.57030 vs 1.151±0.264). In addition, the stress_MPI and MPR of the HAH_28w group were significantly lower than those of the HAH_42w group (0.167±0.058 vs 0.118±0.039 and 1.072±0.581 vs 1.695±0.457). In addition, Masson staining showed inflammatory cell infiltration and collagen deposition in HAH group and the HAH_42w group exhibited more serious pathological changes. The mean fibrosis in HAH_28w group and HAH_42w group were significantly higher than in the NC group (1.53±1.19% vs 0.22±0.08%,P<0.05;15.90±8.95% vs 5.46±5.46%,P<0.01, respectively, Figure 4F). The mean fibrosis in HAH_42w group was significantly higher than in HAH_28w group (P<0.05). Electron microscopy of HAH group cardiac tissue at 28 weeks showed myocardial fiber gap widens, myocardial fiber dissolves, sarcomere atrophy, mitochondrial swelling, moreover, the lumen is filled with host red blood cells, while HAH group cardiac tissue at 42 weeks showed mitochondrial swelling, the myocardial fibers became loose, with the presence of lipid droplets, lysosomes and autophagosomes.Discussion:
In this study, we used the construction of a chronic high-altitude hypoxia HAH experimental group model and a plain NC control group model in a long-term high-altitude environment. The cine and dobutamine stress perfusion sequence comprehensively evaluate the effects of long-term chronic high-altitude hypoxia on the rat heart from the aspects of left and right ventricular function and myocardial first-pass perfusion. The results of blood biochemical examination, histopathology, TUNEL apoptosis immunofluorescence and VEGF immunohistochemistry were analyzed and compared between the HAH experimental group and the NC control group at different times, to explore the multimodal cardiac magnetic resonance imaging, especially the myocardial load. The application value of strain and myocardial perfusion reserve in the early assessment and diagnosis of myocardial damage in chronic high altitude heart disease, and found: 1) High-altitude chronic hypoxic rat myocardium induces myocardial oxygen supply via erythrocytosis and increased myocardial blood flow. 2) First-pass perfusion have certain effects on the early diagnosis of chronic high altitude hypoxia myocardial microcirculation. 3) The stress perfusion index and myocardial reserve index may be the imaging markers to indicate the early myocardial injury of chronic high-altitude hypoxia and to evaluate myocardial microcirculation.Conclusions:
the study determined the feasibility of stress first-pass perfusion CMR to objectively and noninvasively diagnose chronic HAH-induced microvascular changes. This may be of some value in identifying early myocardial changes and understanding the pathophysiology of chronic hypoxia conditions associated with altitude exposure disease.Acknowledgements
We appreciate Yue Jiang’s assistance with the linguistic editing and proofreading of this manuscript. This study was supported in part by the National Natural Science Foundation of China (No. 81930046 and 81829003) and The Expert Workstation of Yunnan Province (No. 202105AF150037), as well as by the Department of Radiology, Medical Imaging of West China Hospital, Sichuan University, Chengdu, Sichuan, China.References
[1]. Parati G, Agostoni P, Basnyat B, et al. Clinical recommendations for high altitude exposure of individuals with pre-existing cardiovascular conditions: A joint statement by the European Society of Cardiology, the Council on Hypertension of the European Society of Cardiology, the European Society of Hypertension, the International Society of Mountain Medicine, the Italian Society of Hypertension and the Italian Society of Mountain Medicine. Eur Heart J. May 1 2018;39(17):1546-1554. doi:10.1093/eurheartj/ehx720
[2]. West JB. High-altitude medicine. Am J Respir Crit Care Med. Dec 15 2012;186(12):1229-37. doi:10.1164/rccm.201207-1323CI
[3]. Murray AJ, Montgomery HE, Feelisch M, Grocott MPW, Martin DS. Metabolic adjustment to high-altitude hypoxia: from genetic signals to physiological implications. Biochem Soc Trans. Jun 19 2018;46(3):599-607. doi:10.1042/bst20170502
[4]. Shao X, Dong X, Cai J, et al. Oxygen Enrichment Ameliorates Cardiorespiratory Alterations Induced by Chronic High-Altitude Hypoxia in Rats. Front Physiol. 2020;11:616145. doi:10.3389/fphys.2020.616145
Figures