4287
The Effect of Resolution and Voxel Size on SNR for Cardiac Diffusion Tensor Imaging1Department of Radiology, Stanford University, Stanford, CA, United States, 2Division of Radiology, Veterans Administration Health Care System, Palo Alto, CA, United States, 3Department of Bioengineering, Stanford University, Stanford, CA, United States, 4Department of Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
Keywords: Heart, Diffusion Tensor Imaging
The purpose of this work is to characterize how SNR varies with slice thickness for a given in-plane resolution for cDTI. We observed that noise in cDTI is largely physiological dominated such that increasing voxel volume yields smaller gains in SNR than what is observed in a phantom. More specifically, increased slice thickness did not significantly improve cDTI metric maps for a given in-plane resolution. However, higher in-plane resolution improved MD and FA maps. Therefore, future work will evaluate the optimal in-plane resolution and slice thickness.Introduction
Cardiac diffusion tensor imaging (cDTI)1 is an MRI technique to probe myocardial mesostructure.2 Despite advancements in cDTI acquisition1,3, it is an open question what optimal acquisition parameters are. cDTI operates in low signal-to-noise (SNR) so to maintain acceptable SNR, diffusion-weighted images have low in-plane resolutions and large through-plane slice thicknesses. This is because if noise contributions are primarily non-physiological (body, coil, or thermal noise), then image SNR increases linearly with voxel volumes. However, in neuroimaging, significant work has characterized physiological noise and the effect of voxel size, demonstrating that in physiological noise-dominated environments, larger volumes are not always proportional to SNR gains.4,5 Isotropic volumes are acquired to minimize sensitivity to through-plane contributions of physiological noise. If cDTI is physiological noise dominated, then thicker slices may result in less SNR gain than theoretically expected compared to thinner slices. This may happen because of incoherent through-plane slice dephasing that produces signal losses, resulting in lower SNR and inaccurate cDTI metrics.The purpose of this work was to characterize the dependence of SNR on different in-plane resolutions and slice thickness for cDTI. Our objective was to find the best combination of SNR and in-plane and through-plane resolutions for a range of cDTI voxel volumes.
Methods
Image AcquisitionHealthy volunteers (n=6, 26.3±3.1 years) were imaged (IRB approved, consented) at 3T MRI (Skyra, Siemens) to obtain a mid-ventricular slice. An ECG-gated and respiratory-triggered, first and second-order motion compensated spin-echo EPI sequence was used to acquire data (TE=84, TR=3×R-R intervals, full-Fourier, GRAPPA=2, b-values=[0,350], 12 diffusion directions, 10 averages, reduced FOV=120×120mm2). The following resolutions were acquired: 2×2×5mm3, 2×2×8mm3, 2.5×2.5×5mm3, 2.5×2.5×8mm3, 3×3×5.5mm3, 3×3×8mm3 (Fig.1). Post-processing included Gibb’s ringing removal, affine image registration, shot rejection, background phase removal6-7, and complex averaging of diffusion-weighted images (DWIs) before computing cDTI metrics. The protocol was acquired in a water phantom for control.
Analysis
Pixel-wise temporal SNR (tSNR) was computed for b=0 and two diffusion directions (in-plane and through-plane). Theoretical and measured percent changes in median SNR between slice thicknesses were compared. Paired t-test of median tSNR between slice thicknesses per in-plane resolution were evaluated (p<0.05). Mean Diffusivity (MD) and Fractional Anisotropy (FA) in the left ventricle (LV) were computed and the median and 95% confidence intervals were reported. Paired t-tests for MD and FA were evaluated between different slice thicknesses for each in-plane resolution and between 2mm and 3mm in-plane resolutions with the same slice thickness (p<0.05).
Results
As expected, the apparent SNR increases with slice thickness in the phantom, (Fig. 2) but does not increase in vivo (Fig. 3).Quantitatively, median tSNR in the phantom significantly (p<0.001) increased 55.2% (theoretical 60%) from changing slice thickness 5 to 8mm for 2mm in-plane resolution, 42.9% (theoretical 60%) increase with changing slice thickness from 5 to 8mm for 2.5 mm in-plane resolution, and 41.1% (theoretical 45%) increase for changing slice thickness from 5.5 to 8mm for 3mm in-plane resolution. In vivo, statistically significant increases in SNR were observed when increasing slice thickness for b=0 images at 2mm (15.5%, p=0.04) and 3mm (13.3%, p=0.03) in-plane resolution. For the in-plane direction, there was a significant increase in tSNR with increasing slice thickness for 2mm resolution (16.4%, p=0.02). For the thru-plane direction, there was a significant increase in tSNR with increasing slice thickness for 2mm (17.3%, p=0.047) and 3mm (35.0%, p=0.03) (Fig.4).
Slice thicknesses for the same in-plane resolution had no discernable differences in MD and FA maps in vivo (Fig. 5). However, there was a significant decrease (p<0.001) in MD from 3mm to 2mm in-plane resolution for both ~5mm slice (1.91 [1.65, 2.17] vs. 1.46 [1.34,1.56]) and 8mm slices (1.88 [1.79,2.01] vs. 1.45 [1.34,1.65]). There was also a significant increase (p=0.3) in FA from 3mm to 2mm in-plane resolution for ~5mm thickness (0.34 [0.25,0.57] vs. 0.40 [0.36,0.68]) and 8mm thickness (0.32 [0.27,0.441] vs. 0.42 [0.35,0.56]).
Discussion
Phantom experiments confirm that our protocol, in a non-physiological noise environment, follows the expected SNR trends. However, we observe that cDTI functions in a physiological noise dominated regime. SNR in vivo increases with slice thickness for a given in-plane resolution, but below theoretical expectations. The lack of this trend in vivo suggests cDTI noise is primarily physiological so increasing slice thickness does not always improve cDTI results. Instead, higher in-plane resolution impacted cDTI accuracy more. Median MD and FA at 2mm resolution trended closer to previous literature8-10 than 3mm in-plane resolutions. This is likely due to larger voxel volumes signal gains being offset by increases in physiological noise.A limitation to this study is that results are dependent upon the protocol. Factors including TE and b-value influence the amount of SNR per voxel volume. Future work will assess the appropriate in-plane resolution and slice thickness combination to be utilized for a given voxel volume. Knowing that higher in-plane resolutions yield improved cDTI metrics, we also plan to test smaller voxel volumes.
Conclusion
We have demonstrated that higher spatial resolutions can improve cDTI metrics and SNR, but further work is needed to define the optimal cDTI spatial resolution for a specific protocol and imaging hardware.Acknowledgements
We would like to acknowledge the following funding sources for supporting this ongoing work: NIH R01 HL131823 to DBE and NSF DGE-1656518 to AJH.References
[1] Nielles-Vallespin S, Khalique Z, Ferreira PF, et al. Assessment of Myocardial Microstructural Dynamics by In Vivo Diffusion Tensor Cardiac Magnetic Resonance. J Am Coll Cardiol. 2017;69(6):661-676. doi:10.1016/j.jacc.2016.11.051
[2] Wilson AJ, Sands GB, LeGrice IJ, Young AA, Ennis DB. Myocardial mesostructure and mesofunction. Am J Physiol Heart Circ Physiol. 2022;323(2):H257-H275. doi:10.1152/ajpheart.00059.2022
[3] Aliotta E, Wu HH, Ennis DB. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion-compensated diffusion-weighted MRI. Magn Reson Med. 2017;77(2):717-729. doi:10.1002/mrm.26166
[4] Triantafyllou C, Hoge RD, Krueger G, et al. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage. 2005;26(1):243-250. doi:10.1016/j.neuroimage.2005.01.007
[5] Krüger G, Glover GH. Physiological noise in oxygenation-sensitive magnetic resonance imaging. Magn Reson Med. 2001;46(4):631-637. doi:10.1002/mrm.1240
[6] Fan Q, Nummenmaa A, Witzel T, et al. Axon diameter index estimation independent of fiber orientation distribution using high-gradient diffusion MRI. Neuroimage. 2020;222:117197. doi:10.1016/j.neuroimage.2020.117197
[7] Scott AD, Nielles-Vallespin S, Ferreira PF, McGill LA, Pennell DJ, Firmin DN. The effects of noise in cardiac diffusion tensor imaging and the benefits of averaging complex data. NMR Biomed. 2016;29(5):588-599. doi:10.1002/nbm.3500.
[8] Aliotta E, Moulin K, Magrath P, Ennis DB. Quantifying precision in cardiac diffusion tensor imaging with second-order motion-compensated convex optimized diffusion encoding. Magn Reson Med. 2018;80(3):1074-1087. doi:10.1002/mrm.27107.
[9] Moulin K, Verzhbinsky IA, Maforo NG, Perotti LE, Ennis DB. Probing cardiomyocyte mobility with multi-phase cardiac diffusion tensor MRI. PLoS One. 2020;15(11):e0241996. Published 2020 Nov 12. doi:10.1371/journal.pone.0241996
[10] Moulin K, Croisille P, Viallon M, Verzhbinsky IA, Perotti LE, Ennis DB. Myofiber strain in healthy humans using DENSE and cDTI. Magn Reson Med. 2021;86(1):277-292. doi:10.1002/mrm.28724
Figures
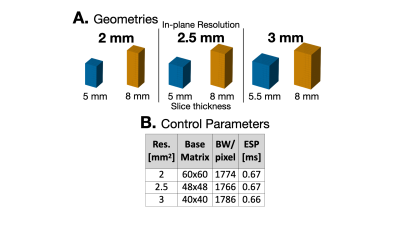
Figure 1. Overview of experimental design. (A) Provides example of the voxel geometries that were evaluated for each in-plane resolution. Compared to the thinner slice thickness (blue), the thicker slices (orange) may be more sensitive to physiological noise. (B) Acquisition parameters highlight the difference in base matrix size for the different resolutions. Bandwidth and echo-spacing were kept as close as possible in order for images to have similar geometric distortions and to closely match imaging parameters.
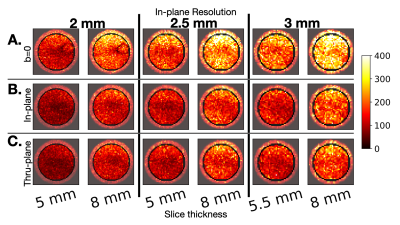
Figure 2. The tSNR experimental results in the water phantom. For (A) the b=0 non-DWIs, we observe higher tSNR than the DWIs (B-C). As expected, we observe that tSNR increases with slice thickness both for DWIs with diffusion-encoding along (B) in-plane direction and (C) through-plane direction. These trends are aligned with previous literature and demonstrate how tSNR scales linearly with increasing slice thickness in a predominiately thermal (non-physiologic) noise enviornment.
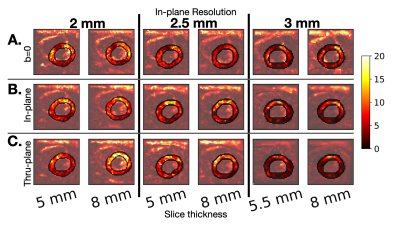
Figure 3. Example of tSNR maps for one volunteer for (a) b=0 non-DWIs, (b) DWIs with diffusion encoding in-plane and (c) DWIs with diffusion encoding through-plane. We observe that tSNR does not necessarily increase with slice thickness for a given in-plane resolution in cDTI. tSNR maps vary over the LV, with higher tSNR in the mid anterior wall versus the mid inferolateral wall.
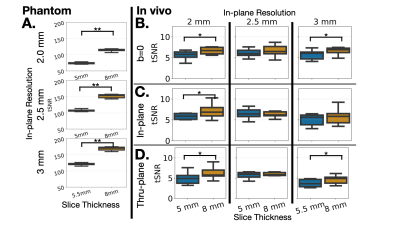
Figure 4. (A) Median tSNR for the phantom from 12 diffusion directions in a boxplot, demonstrating that increasing slice thickness increases SNR for all in-plane resolutions, scaling as expected. Median tSNR for the 6 volunteers represented as boxplots for (B) non-DWIs, (C) DWIs for the in-plane direction (D) DWIs for the through-plane direction. Smaller SNR gains in vivo compared to theory. Statistical significance indicated by stars (**<0.001,*< 0.5).
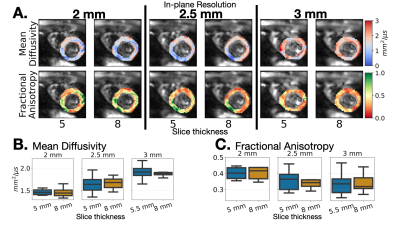
Figure 5. (A) Exemplary MD and FA maps for one volunteer. Elevated MD is observed at 3 mm which decreases for 2.5 and 2mm. Boxplots of median (B) MD and (C) FA across volunteers for the two slice thicknesses per in-plane resolution. No statistical significance between slice thicknesses for a given in-plane resolution. In-plane resolution changes have more impact on MD and FA than altering slice thicknesses as MD decreases and FA increases at 2mm vs. 3mm.