4286
Structural alterations in right ventricle of hypoxic pulmonary hypertensive rats assessed by high-resolution DTI1IHU Liryc, Bordeaux, France, 2CRCTB U1045, Bordeaux, France, 3CRMSB UMR 5536, Bordeaux, France
Synopsis
Keywords: Cardiomyopathy, Hypertension
Transgenic rats with Bmpr2 mutation is a model of heritable pulmonary arterial hypertension. This study aims to evaluate structural alterations of Bmpr2-mutants associated with a risk factor (chronic hypoxia) by high-resolution diffusion tensor imaging (DTI).
Chronic increases of pressure-loading conditions appears to be poorly tolerated by the right ventricle (RV). Indeed, chronic hypoxia groups showed a hypertrophy in RV. Moreover, DTI analysis showed disturbances in RV (myofibers and sheetlets) and this more prominently at basal level in transgenic rats. Consequently, hypoxic pulmonary hypertension can induce microstructural changes and electrophysiological remodeling, which are potentiated by Bmpr2 mutation.
Introduction
Pulmonary arterial hypertension (PAH) is characterized by an increase in mean pulmonary arterial pressure (mPAP) causing right ventricular structural changes along with electrophysiological remodeling which lead to arrhythmias1. Heritable form of PAH is mostly associated to mutations in bone morphogenic protein receptor 2 (Bmpr2) gene. Patient’s carriers of Bmpr2 mutations are diagnosed younger and showed more severe clinical features2. Penetrance of PAH in Bmpr2 mutation carriers is generally around 20% suggesting secondary factors contribute to make the disease clinically deleterious3. Indeed, the severity of PAH phenotype in Bmpr2 carriers is also influenced by hypoxia who showed an increased susceptibility to hypoxic pulmonary vasoconstriction4,5.In this study, experiments were performed on the first rat model with a monoallelic mutation of Bmpr2 which showed a penetrance similar as Bmpr2 patients6 and it was combine with chronic hypoxia to exacerbate PAH. Furthermore, Bmpr2 gene is expressed in the right ventricle (RV) and it was hypothesized that RV function is the main determinant in the clinical phenotypes of mutation carriers and noncarriers7. Previous study reported an increase in mPAP and a drastic dilatation of the RV in transgenic animals under chronic hypoxia8.
Using ex vivo diffusion tensor imaging (DTI), our objective was to evaluate if Bmpr2 mutation can exacerbate cardiac structural alterations in Bmpr2 rats exposed to chronic hypoxia.
Methods
Male Sprague-Dawley rats aged of 3-month-old with transgenic genotype (TG, Bmpr2 monoallelic mutation) or wild type genotype (WT, non-mutated brothers) were divided in two conditions (n=3/group): normoxia and chronic hypoxia (PO2 in inspired air of 79.8 mmHg for 3 weeks). After sacrifices of the rats, the hearts were excised and then retrogradely perfused with an ice-cold modified Krebs-Henseleit solution on a Langendorff system. Blebbistatin was used to perform electro-mechanical uncoupling which blocked the hearts in diastolic phase of the cardiac cycle. The hearts were then fixed with a formalin solution with 0.1% of contrast agent (gadoterate of meglumine). Prior to MRI experiments, hearts were immerged in fluorinert (perfluropolyether with no MR signal to avoid susceptibility artifacts) (Fig.1).MR acquisitions were performed on a 9.4T Biospec MRI (Bruker, Wissembourg, France) of 20 cm aperture. A transmit-quadrature 1H coil coupled with phase array coil in reception was used for MRI. 3D spin echo DTI sequence parameters: TE/TR= 23/500ms; δ/Δ = 4.35/11ms; b-value=1000 s/mm²; 6 directions; field of view= 30x30x30 mm; resolution= 200 µm3 isotropic; 2 averages; acquisition time= 31h7m30s. DT images were used to measure wall thickness, fractional anisotropy (FA), mean diffusivity (MD), eigenvalues (λ1, λ2, λ3) in the left ventricle (LV) and the right ventricle (RV) and into basal, mid-ventricular and apex slices (Paravision 6.0, Bruker) (Fig.2). Eigenvalue 1 represents the organization of the myofibers, eigenvalue 2 shows the intermediary orientation and eigenvalue 3 indicates the sheetlets organization. Groups were compared by non-parametric Kruskal-Wallis test followed by a post-hoc test of Dunn with *p<0.05; **p<0.01 (GraphPad Prism 9.2).
Results
Firstly, wall thickness was measured in LV and RV to assess the hypertrophy status of cardiac ventricles in rats exposed to chronic hypoxia. No changes was measured in LV (Fig.3A) but there is a significant increase in RV at basal, mid and apex levels in rats (WT and TG) exposed to chronic hypoxia (Fig.3B).Secondly, diffusion tensor parameters were assessed in LV and RV at basal, mid and apex levels in order to evaluate the 3D microstructural organization of cardiac tissue.
DT parameters for LV are summarized in Fig.4. A significant decrease of FA was measured in rats (WT and TG) exposed to chronic hypoxia along with an increase in MD. These variations can be explained by an elevation in eigenvalues 2 and 3 indicating a disorganization in the sheetlets.
DT measurements for RV are presented in Fig.5. FA showed no changes in normoxia groups but a decline in rats exposed to chronic hypoxia, and this more prominently at basal level in TG rats. Inversely as FA, MD is increased in chronic hypoxia groups related to an elevation in all three eigenvalues at basal and mid-ventricular position. In chronic hypoxia groups, myofibers and sheetlets organization is modified. Interestingly, there is also an increase in eigenvalues 1 and 2 in RV of TG-normoxia group compared to WT-normoxia group, which seems to indicate that Bmpr2 mutation disturbs myofibers organization.
Conclusion
Transgenic rat model of PAH under normoxia condition showed an alteration in myofibers organization of RV, which could be of clinical interest for early diagnosis of the pathology. Electrophysiological analysis of the same hearts measured a longer action potential duration (APD) in RV (data not shown).Chronic increases of pressure-loading conditions PAH model (reproduced here using hypoxic conditions) appears to be poorly tolerated by the RV. Chronic hypoxia groups showed a hypertrophy in RV although no change in LV wall thickness was observed. Moreover, DTI analysis showed disturbances in RV (myofibers and sheetlets), more prominently at basal level in TG rats, in agreement with electrophysiological studies (elongated APD in RV of chronic hypoxia groups with an extended effect in TG rats). Consequently, hypoxic pulmonary hypertension induce microstructural changes and electrophysiological remodeling which are potentiated by Bmpr2 mutation.
Acknowledgements
This study received financial support from the French Government as part of the “Investments of the Future” program managed by the National Research Agency (ANR), Grant reference ANR-10-IAHU-04. This project also received financial support from the French Federation of Cardiology (FFC).References
1. Cirulis, M. M., Ryan, J. J. & Archer, S. L. Pathophysiology, incidence, management, and consequences of cardiac arrhythmia in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Pulm. Circ. 9, 204589401983489 (2019).2. Evans, J. D. W. et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir. Med. 4, 129–137 (2016).
3. Maron, B. A. & Loscalzo, J. Pulmonary Hypertension: Pathophysiology and Signaling Pathways. in Pharmacotherapy of Pulmonary Hypertension (eds. Humbert, M., Evgenov, O. V. & Stasch, J.-P.) vol. 218 31–58 (Springer Berlin Heidelberg, 2013).
4. Pavelescu, A., Vanderpool, R., Vachiéry, J.-L., Grunig, E. & Naeije, R. Echocardiography of pulmonary vascular function in asymptomatic carriers of BMPR2 mutations. Eur. Respir. J. 40, 1287–1289 (2012).
5. Claessen, G. et al. Right ventricular and pulmonary vascular reserve in asymptomatic BMPR2 mutation carriers. J. Heart Lung Transplant. 36, 148–156 (2017).
6. Hautefort, A. et al. Bmpr2 Mutant Rats Develop Pulmonary and Cardiac Characteristics of Pulmonary Arterial Hypertension. Circulation 139, 932–948 (2019).
7. van der Bruggen, C. E. et al. Bone Morphogenetic Protein Receptor Type 2 Mutation in Pulmonary Arterial Hypertension: A View on the Right Ventricle. Circulation 133, 1747–1760 (2016).
8. El Hamrani, D. et al. Characterization of cardiac alterations in transgenic rat model of pulmonary arterial hypertension exposed to chronic hypoxia. ISMRM, London, UK; 7-12 may 2022.
Figures
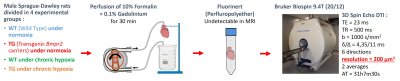
Figure 1: Schematic representation of experimental protocol
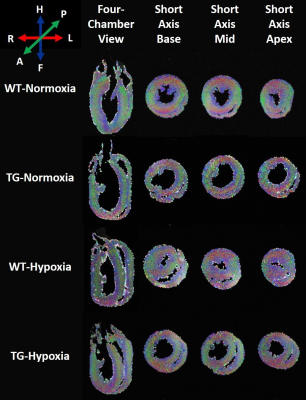
Figure 2: Representative diffusion tensor images in four-chamber and short-axis views obtained in wild-type (WT) and transgenic (TG) rats under normoxia and chronic hypoxia (resolution 200µm3 isotropic). Color coding is red for transverse fibers (right-left), green for anteroposterior fibers and blue for craniocaudal fibers (head-feet).
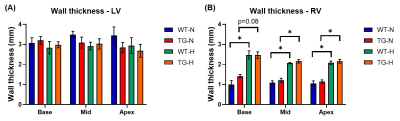
Figure 3: Wall thickness of (A) left ventricle (LV) and (B) right ventricle (RV) obtained at basal, mid-ventricular and apex in wild-type (WT) and transgenic (TG) rats under normoxia (N) and chronic hypoxia (H). Groups were compared by non-parametric Kruskal-Wallis test followed by a post-hoc test of Dunn with *p<0.05.
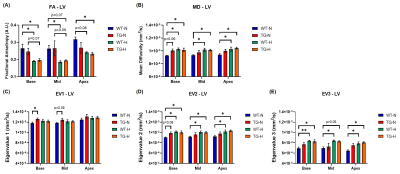
Figure 4: Diffusion tensor parameters of left ventricle (LV) obtained at basal, mid-ventricular and apex in wild-type (WT) and transgenic (TG) rats under normoxia (N) and chronic hypoxia (H): (A) fractional anisotropy, (B) mean diffusivity, (C) eigenvalue 1, (D) eigenvalue 2 and (E) eigenvalue 3. Groups were compared by non-parametric Kruskal-Wallis test followed by a post-hoc test of Dunn with *p<0.05 and **p<0.01.
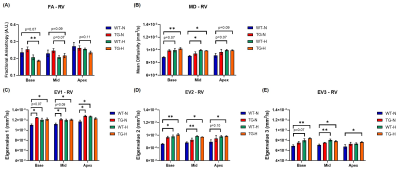
Figure 5: Diffusion tensor parameters of right ventricle (RV) obtained at basal, mid-ventricular and apex in wild-type (WT) and transgenic (TG) rats under normoxia (N) and chronic hypoxia (H): (A) fractional anisotropy, (B) mean diffusivity, (C) eigenvalue 1, (D) eigenvalue 2 and (E) eigenvalue 3. Groups were compared by non-parametric Kruskal-Wallis test followed by a post-hoc test of Dunn with *p<0.05 and **p<0.01.