4284
Cardiac diffusion MRI using Connectom scanner1Leeds Institute of Cardiovascular and Metabolic Medicine, Leeds, United Kingdom, 2Cardiff University Brain Research Imaging Centre (CUBRIC), School of Psychology, Cardiff University, Cardiff, United Kingdom, 3These authors contributed equally to this work, Leeds, United Kingdom, 4Siemens Healthcare Ltd, Camberly, United Kingdom, 5Siemens Healthcare GmbH, Erlangen, Germany, 6Medical Radiation Physics, Clinical Sciences Lund, Lund University, Lund, Sweden
Synopsis
Keywords: Heart, Diffusion Tensor Imaging, Strong gradients
Cardiac diffusion Magnetic Resonance Imaging (cardiac dMRI) allows to non-invasively assess the microstructure of the heart. Cardiac motion typically necessitates the use of motion-compensated diffusion gradients. Here we report for the first time on the application of cardiac dMRI on a Connectom MR scanner with a gradient strength of 300 mT/m using a spin echo (SE) sequence with EPI readout and up to third order (M3) motion-compensated diffusion gradients. The results show the benefits of applying dMRI with higher order motion compensation in the human heart using a Connectom scanner.Introduction
Cardiac diffusion-weighted imaging is one of the most challenging MR imaging techniques, which is confounded by macroscopic motion. Spin-echo (SE) with motion-compensated diffusion gradients can abate this challenge. A first-order motion-compensated approach was proposed by Gamper et al. in1. More recently, this approach was extended by Stoeck et al.2 to compensate motion up to second order (M2-nulling), i.e. constant velocity and acceleration are both nulled. While jerk-nulling (M3-nulling) was explored in rat heart3, second-order motion compensation remains the standard for spin echo-based cardiac diffusion tensor imaging (cDTI) in human hearts. Most efforts to improve the SNR of dMRI aim to limit the signal loss due to T2 decay4. On the hardware side, this is best achieved by stronger gradients. In this work, we show the feasibility of cardiac diffusion-weighted imaging with third-order motion compensation using the Connectom scanner.Methods
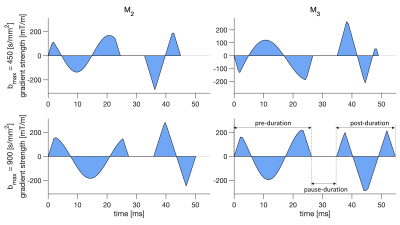
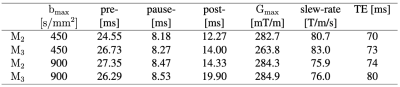
We acquired cardiac dMRI on a Connectom 3T research-only MR imaging system (Siemens Healthcare, Erlangen, Germany) with a gradient strength of 300 mT/m and a slew rate of 200 T/m/s. Data were acquired from five healthy volunteers who provided written consent. Optimizing gradient performance with respect to maximum amplitude and slew rate needs to consider PNS5,6. Therefore, we carefully designed up to order 3 (M2 and M3) motion-compensated gradient waveforms with a maximum gradient strength of 285 mT/m and a maximum slew rate of 80 T/m/s using the NOW toolbox7 (Figure 1).We used routine GRE and TRUEFISP sequences for cardiac planning and cine-imaging, whereas dMRI was performed with a prototype pulse sequence that enables diffusion encoding with user-defined gradient waveforms8. The imaging parameters were: TR = 3RR-intervals, field‐of‐view = 320 × 320 mm2, in‐plane resolution = 2.3 × 2.3 mm2, slice thickness = 8 mm, 3 short axis slices (base, mid, and apical), partial Fourier = 6/8, no parallel imaging, bandwidth = 2012 Hz/pixel. We used two bmax values, bmax = 450 s/mm2 which is commonly used in clinical scanners for cardiac diffusion MRI and twice this value which is bmax = 900 s/mm2 for both M2 and M3. The first diffusion-weighted imaging scheme includes b = 100 and 450 s/mm2 in 3 and 30 directions respectively with 6 repetitions while the second scheme includes b = 100, 450, and 900 s/mm2 in 10, 30, and 30 directions respectively with two repetitions. The total acquisition time was one hour. The details of the experiment design are given in Figure 2.
Results
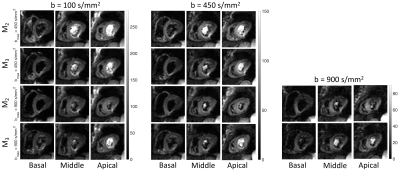
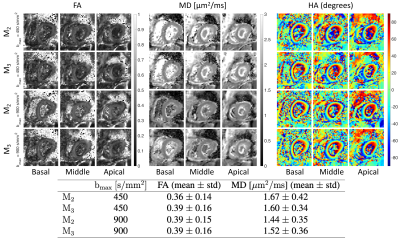
Figure 3 shows the cardiac diffusion-weighted images acquired in basal, mid, and apical slices with b = 100, 450, and 900 s/mm2 using four different acquisition schemes.Figure 4 shows the estimated fractional anisotropy (FA), mean diffusivity (MD), and helix angle (HA) from data acquired using second and third-order motion compensated waveforms (M2 and M3) with bmax = 450 s/mm2 and bmax = 900 s/mm2 for three different slices.
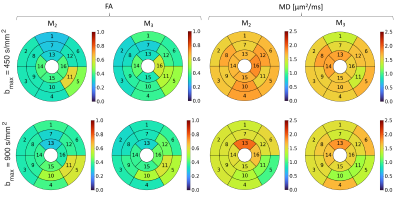
Figure 5 shows the bullseye plots for FA and MD corresponding to each segment of the American heart association (AHA) model using second and third-order motion compensated waveform (M2 and M3) with bmax = 450 s/mm2 and bmax = 900 s/mm2. The values are consistent between different acquisitions and we do not observe any significant difference between the plots visually. It shows the feasibility of acquiring cardiac dMRI data with reasonable quality using the Connectom scanner.
Discussion
Second-order motion compensation is commonly used to compensate the acceleration and velocity. Third-order compensation needs a long echo time (TE), however using a Connectom scanner, the minimum TE for bmax = 450 s/mm2 is 73 ms which is of the order of the echo time that is used in clinical scanners for second-order motion compensated waveforms9,10. Note that we do not have the Zoom-IT pulse available on the Connectom and so we need to acquire a larger FoV which makes the echo time longer.On the hardware side, strong gradients can help to reduce the echo time and therefore improve the SNR in diffusion-weighted images. Available gradient amplitude in Connectom is one key determinant of TE because diffusion gradients tend to be substantially longer than gradient ramp times even at moderate slew rates. For EPI, the slew rate is also very important, which is why in some cases head only gradients with lower Gmax but higher slew can outperform connectom on TE. High-amplitude gradients with high duty cycle are thus prime means of improving the SNR in dMRI6,11.
Our future work is to use spiral readout12,13 to reduce the echo time in cardiac diffusion-weighted imaging using a Connectom scanner. To the best of our knowledge, this is the first experiment conducted using a human Connectom scanner for cardiac dMRI.
Conclusion
Gradient strength and acquisition efficiency are critical for sensitivity in diffusion imaging. Encouraged by the knowledge that Connectom scanners with high gradient strength appeared to produce richer information than is possible using clinical scanners, in brain studies, we explored the use of this for cardiac dMRI for the first time.Acknowledgements
We thank Siemens Healthcare for the pulse sequence development environment. This work was supported by Wellcome Trust Investigator Award (219536/Z/19/Z), EPSRC (EP/M029778/1), and The Wolfson Foundation.References
1. Gamper U, Boesiger P, Kozerke S. Diffusion imaging of the in vivo heart using spin echoes–considerations on bulk motion sensitivity. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2007 Feb;57(2):331-7.
2. Stoeck CT, Von Deuster C, Genet M, Atkinson D, Kozerke S. Second‐order motion‐compensated spin echo diffusion tensor imaging of the human heart. Magnetic resonance in medicine. 2016 Apr;75(4):1669-76.
3. Welsh CL, DiBella EV, Hsu EW. Higher-order motion-compensation for in vivo cardiac diffusion tensor imaging in rats. IEEE transactions on medical imaging. 2015 Mar 9;34(9):1843-53.
4. Lee Y, Wilm BJ, Brunner DO, Gross S, Schmid T, Nagy Z, Pruessmann KP. On the signal‐to‐noise ratio benefit of spiral acquisition in diffusion MRI. Magnetic resonance in medicine. 2021 Apr;85(4):1924-37.
5. Reilly JP. Peripheral nerve stimulation by induced electric currents: exposure to time-varying magnetic fields. Medical and Biological Engineering and Computing. 1989 Mar;27(2):101-10.
6. Setsompop K, Kimmlingen R, Eberlein E, Witzel T, Cohen-Adad J, McNab JA, Keil B, Tisdall MD, Hoecht P, Dietz P, Cauley SF. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. Neuroimage. 2013 Oct 15;80:220-33.
7. Sjölund J, Szczepankiewicz F, Nilsson M, Topgaard D, Westin CF, Knutsson H. Constrained optimization of gradient waveforms for generalized diffusion encoding. Journal of magnetic resonance. 2015 Dec 1;261:157-68.
8. Szczepankiewicz F, Sjölund J, Ståhlberg F, Lätt J, Nilsson M. Tensor-valued diffusion encoding for diffusional variance decomposition (DIVIDE): Technical feasibility in clinical MRI systems. PLoS One. 2019 Mar 28;14(3):e0214238.
9. Szczepankiewicz F, Sjölund J, Dall’Armellina E, Plein S, Schneider JE, Teh I, Westin CF. Motion‐compensated gradient waveforms for tensor‐valued diffusion encoding by constrained numerical optimization. Magnetic resonance in medicine. 2021 Apr;85(4):2117-26.
10. Lasič S, Szczepankiewicz F, Dall'Armellina E, Das A, Kelly C, Plein S, Schneider JE, Nilsson M, Teh I. Motion‐compensated b‐tensor encoding for in vivo cardiac diffusion‐weighted imaging. NMR in Biomedicine. 2020 Feb;33(2):e4213.
11. Weiger M, Overweg J, Rösler MB, Froidevaux R, Hennel F, Wilm BJ, Penn A, Sturzenegger U, Schuth W, Mathlener M, Borgo M. A high‐performance gradient insert for rapid and short‐T2 imaging at full duty cycle. Magnetic resonance in medicine. 2018 Jun;79(6):3256-66.
12. Wilm BJ, Hennel F, Roesler MB, Weiger M, Pruessmann KP. Minimizing the echo time in diffusion imaging using spiral readouts and a head gradient system. Magnetic resonance in medicine. 2020 Dec;84(6):3117-27.
13. Mueller L, Afzali M, Molendowska M, Tax C, Fasano F, Rudrapatna S, et al. Boost-ing the SNR-efficiency of Free Gradient Waveform Diffusion MRI using SpiralReadouts and Ultra-Strong Gradients. In: Proceedings of the 29th Annual Meeting of ISMRM; 2021.
Figures