4283
Opportunities for improved myocardial first-pass perfusion imaging at 0.55T1Ming Hsieh Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Siemens Medical Solutions, Los Angeles, CA, United States, 3Division of Cardiovascular Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
Synopsis
Keywords: Myocardium, Perfusion
Myocardial first-pass perfusion (FPP) MRI is used in the clinic to diagnose coronary artery disease but has limited spatial resolution and coverage. Contemporary low-field scanners have the potential to provide FPP imaging with improved resolution and coverage by leveraging different acquisitions, such as spiral bSSFP. In this study, we demonstrate a time-efficient spiral bSSFP FPP pulse sequence at 0.55T and compare it with the standard Cartesian FPP in 4 healthy volunteers. Measured SNR, CNR, and myocardial boundary sharpness scores are all higher in the spiral images than in Cartesian images.Introduction
Myocardial first-pass perfusion (FPP) MRI is a useful tool for the diagnose of coronary artery disease. There has also been increasing interest in applying cardiac MRI on contemporary 0.55T scanners1-4. Such systems have reduced off-resonance, different intrinsic relaxation properties (shorter T1 and longer apparent T2), and relaxed SAR constraint, which give greater latitude in pulse sequence design and the possibility of improved image quality. Non-Cartesian sampling provides higher acquisition efficiency and can simultaneously provide finer spatial resolution and broader coverage5-7, and is favorable at low field2.In this study, we implemented a saturation recovery spiral bSSFP pulse sequence for myocardial FPP, that provides high-resolution myocardial perfusion images without any acceleration, and compared it with conventional Cartesian FPP in healthy volunteers at rest.
Methods
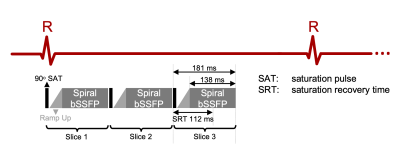
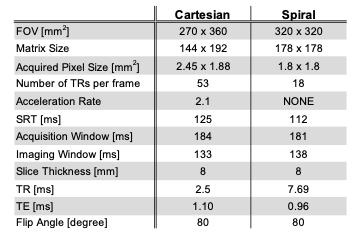
Pulse Sequence: Figure 1 illustrates the spial bSSFP pulse sequence. The sequence was implemented using the RTHawk (HeartVista, Inc., Menlo Park, CA) scanning platform8. The sequence images three slices per heartbeat, with each slice comprised of a non-selective saturation recovery preparation9 and 2D spiral bSSFP readout. The Cartesian sequence uses the same saturation recovery pulse followed by 2D Cartesian bSSFP with parallel imaging acceleration. Detailed scan parameters are listed in Table 1.Protocol: Experiments were performed using a whole body 0.55T system (prototype MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany) equipped with high-performance shielded gradients (45 mT/m amplitude, 200 T/m/s slew rate). Four healthy volunteers (age 21-25, 2 female) were enrolled in this study, under a protocol approved by our Institutional Review Board, after providing written informed consent. The imaging protocol includes a cardiac localizer, pre-contrast T1 mapping, rest FPP, whole-heart cine, rest FPP, post-contrast T1 mapping and LGE. In each FPP scan, 0.05mmol/kg (half dose) of gadobenate dimeglumine (MultiHance, Bracco Diagnostics, Milan, Italy) was manually injected through IV at a rate of 3-5ml/s following by a 20ml saline flush. ECG-gated FPP sequence acquired data for 60-100 heartbeats in three short-axis slices covering the basal, mid, and apical LV myocardium. In each subject, two injections were performed with a ~10-minute gap in between. In two subjects, we ran the Cartesian FPP first, and in the other two subjects we ran the spiral FPP first. The total scan time per subject was ≤ 1 hour.
Evaluation: SNR units reconstruction10 was performed offline with CG-SENSE for spiral and GRAPPA for Cartesian. SNR, CNR, and myocardial wall sharpness score as described by Lim et al.11 were calculated. SNR was calculated and reported for three time points: 1) baseline LV blood and myocardium, 2) peak LV blood enhancement, and 3) peak LV myocardium enhancement. The myocardium was segmented according to the 17-segment model12 and SNR was calculated in each segment and averaged across 16 out of 17 segments (3 short-axis slices). CNR was calculated between the peak and baseline LV blood and myocardium, respectively. Boundary sharpness scores were calculated from each cardiac segment, including endocardial and epicardial boundaries. To obtain signal enhancement curves, images were registered to a low-rank approximation with similar image contrast13 and using LV blood and myocardium contours.
Results
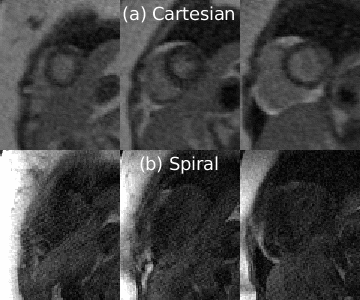
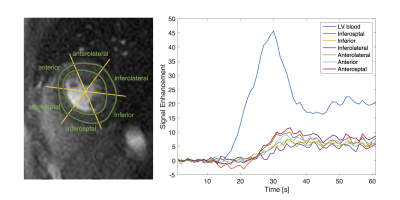
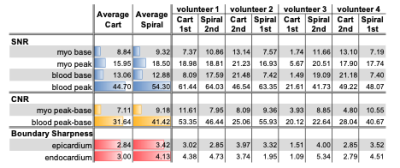
Figure 2 shows perfusion images from one representative subject where spiral FPP was performed first. Both spiral and Cartesian images capture the contrast dynamics clearly; we can see the contrast enhancement in the RV blood, LV blood, and myocardium, and all myocardial boundaries are clearly depicted. We observe a small dark rim artifact in the middle slice of the Cartesian images but not on the spiral images. The spiral images appear sharper, largely due to the reduced voxel size. Figure 3 shows the signal enhancement curves from the mid-ventricular spiral image of another representative subject. We observe the signal curves in the myocardium enhance uniformly, as is expected for healthy volunteers.Table 2 reports the quantitative SNR, CNR, and boundary sharpness scores. Spiral and Cartesian images have comparable baseline SNR in both the LV blood and myocardium. The peak myocardial and blood SNR, as well as the CNR are higher in the spial images. The boundary sharpness scores are also higher in the spiral images.
Discussion
Compared with the conventional Cartesian FPP images, the experimental spiral FPP images generally had finer spatial resolution, higher SNR, CNR, and boundary sharpness scores. We were surprised that Cartesian FPP had lower SNR, despite having significantly larger voxel size. This could be caused by the need for parallel imaging for cartesian imaging, and/or the fact that the spiral acquisition samples more densely at the k-space origin. Spiral FPP had a higher boundary sharpness, which is not surprising, since the voxel dimensions were significantly smaller.Future studies are needed to compare 0.55T spiral FPP sequence against a conventional field strength (1.5T or 3T) reference, and to determine the feasibility of perfusion quantification. Future studies also need to evaluate the spiral FPP in patients with known CAD. Combining the spiral sequence with simultaneous multi-slice or 3D acquisitions6,7,14,15 is also of interest as a means to provide greater spatial coverage.
Conclusion
We have demonstrated the feasibility of performing myocardial first-pass perfusion MRI at 0.55T with a time efficient spiral bSSFP. This approach simultaneously provides superior spatial resolution, SNR, CNR, and boundary sharpness compared to conventional Cartesian counterparts.Acknowledgements
We acknowledge grant support from AHA Grant #903839, NIH R21-HL159533, and NSF Grant #1828736. We also acknowledge research support from Siemens Healthineer. We thank Mary Yung for research coordination and Elizabeth Arevalo for nursing support.References
1. Bandettini WP, Shanbhag SM, Mancini C, McGuirt DR, Kellman P, Xue H, et al. A comparison of cine CMR imaging at 0.55 T and 1.5 T. Journal of Cardiovascular Magnetic Resonance. 2020;22(1):37.
2. Restivo MC, Ramasawmy R, Bandettini WP, Herzka DA, Campbell-Washburn AE. Efficient spiral in-out and EPI balanced steady-state free precession cine imaging using a high-performance 0.55T MRI. Magnetic Resonance in Medicine. 2020;84(5):2364-75.
3. Campbell-Washburn AE, Ramasawmy R, Restivo MC, Bhattacharya I, Basar B, Herzka DA, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology. 2019;293(2):384-93.
4. Tian Y, Cui SX, Lim Y, Lee NG, Zhao Z, Nayak KS. Contrast-optimal simultaneous multi-slice bSSFP cine cardiac imaging at 0.55 T. Magn Reson Med. 2022.
5. Wang J, Yang Y, Weller DS, Zhou R, Van Houten M, Sun C, et al. High spatial resolution spiral first-pass myocardial perfusion imaging with whole-heart coverage at 3 T. Magnetic Resonance in Medicine. 2021;86(2):648-62.
6. Tian Y, Mendes J, Wilson B, Ross A, Ranjan R, DiBella E, et al. Whole-heart, ungated, free-breathing, cardiac-phase-resolved myocardial perfusion MRI by using Continuous Radial Interleaved simultaneous Multi-slice acquisitions at sPoiled steady-state (CRIMP). Magn Reson Med. 2020.
7. Yang Y, Meyer CH, Epstein FH, Kramer CM, Salerno M. Whole-heart spiral simultaneous multi-slice first-pass myocardial perfusion imaging. Magn Reson Med. 2019;81(2):852-62.
8. Santos JM, Wright GA, Pauly JM. Flexible real-time magnetic resonance imaging framework. Conf Proc IEEE Eng Med Biol Soc. 2004;2004:1048-51.
9. Kim D, Gonen O, Oesingmann N, Axel L. Comparison of the effectiveness of saturation pulses in the heart at 3T. Magn Reson Med. 2008;59(1):209-15.
10. Kellman P, McVeigh ER. Image reconstruction in SNR units: A general method for SNR measurement†. Magnetic Resonance in Medicine. 2005;54(6):1439-47.
11. Lim Y, Lingala SG, Narayanan SS, Nayak KS. Dynamic off-resonance correction for spiral real-time MRI of speech. Magn Reson Med. 2019;81(1):234-46.
12. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18(1):539-42.
13. Xue H, Brown LAE, Nielles-Vallespin S, Plein S, Kellman P. Automatic in-line quantitative myocardial perfusion mapping: Processing algorithm and implementation. Magn Reson Med. 2020;83(2):712-30.
14. Tian Y, Mendes J, Pedgaonkar A, Ibrahim M, Jensen L, Schroeder JD, et al. Feasibility of multiple-view myocardial perfusion MRI using radial simultaneous multi-slice acquisitions. Plos One. 2019;14(2).
15. Mendes JK, Adluru G, Likhite D, Fair MJ, Gatehouse PD, Tian Y, et al. Quantitative 3D myocardial perfusion with an efficient arterial input function. Magn Reson Med. 2019.
Figures