4278
VI-RADS combined with decision tree model preoperatively predict the pathological grade of bladder cancer with high (≥3) and low (≤2) scores1Zhongshan Hospital, Fudan University, Shanghai, China, 2MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Urogenital, Bladder
Bladder cancer pathology grading is currently obtained mainly by invasive cystoscopic biopsy or surgical pathology. This study aimed to investigate the effectiveness of Vesical Imaging Reporting and Data System (VI-RADS) in the diagnosis of high-grade bladder cancer (HG-BC) and explore a new preoperative non-invasive grading prediction system. We demonstrate that most bladder cancers with a VI-RADS score ≥3 are high-grade and that the decision tree model is a good predictor of pathological grading in patients with VI-RADS ≤2. Thus, VI-RADS can be a grouping imaging biomarker for noninvasive prediction of bladder cancer grade.Introduction
High-grade bladder cancer (HG-BC) is characterized by relatively high rates of recurrence and progression 1,2. Therefore, pre-treatment grading for the effective diagnosis of BC is significant for patients. At present, cystoscopic biopsy and surgery such as diagnostic transurethral resection of bladder tumor remain the gold standard for the diagnosis and pathological grading of BC 3,4. Hence, an accurate and noninvasive grading method before treatment is crucial for personal management. VI-RADS has been demonstrated to be efficient in detecting MIBC in primary patients without exploring its predictive efficacy of pathological grade of BC 5-7. This study aimed to explore the diagnostic efficiency of VI-RADS for predicting HG-BC and to construct a preoperative noninvasive grading method based on VI-RADS.Methods
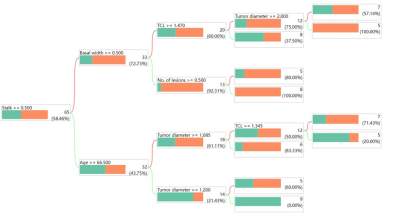
We prospectively enrolled 89 patients with primary BC who underwent preoperative multiparametric magnetic resonance imaging. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of VI-RADS for predicting HG-BC and MIBC in the entire group. The low VI-RADS (≤2) group (n=65) was isolated from the entire cohort. In the subgroup, the decision tree-based method was used to obtain significant predictors and construct the decision tree model (DT-model). The diagnostic flowchart of DT-model is shown in Figure 1. In the low VI-RADS group, the performance of the DT-model and low VI-RADS scores for predicting HG-BC was determined using ROC curve, calibration curve and decision curve analyses.Results
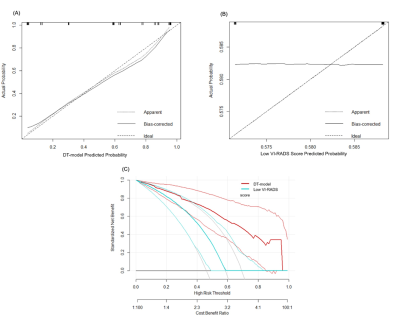
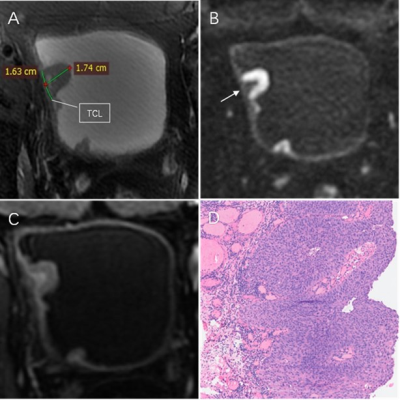
A total of 89 patients (median age, 69 years; interquartile range, 64-75 years; 70 male) were enrolled. At cut-off ≥3, the specificity and positive predictive value of VI-RADS for predicting HG-BC in the whole group were 100.0%, the AUC was 0.697. For VI-RADS prediction of MIBC in the entire group, the AUC was 0.965. Among 65 patients with low VI-RADS score, the DT-model showed an AUC of 0.884 in predicting HG-BC compared with 0.506 for low VI-RADS scores. The calibration curve and the decision curve analyses showed that the DT-model performed better than the low VI-RADS score. The calibration and decision curves of the subgroup are shown in Figure 2. Case examples of VI-RADS scoring and the DT-model within HG-BC patients are shown in Figure 3 and 4.Discussion
This study indicates that a high VI-RADS score (≥3) has a good positive predictive value for the diagnosis of HG-BC, and that most MIBCs are HG. This is consistent with the guidelines that >95% of invasive tumors are HG, and a small percentage of invasive carcinomas are low-grade, usually limited to the lamina propria8. Since the vast majority of MIBCs are HG-BC, the necessity of including patients with MIBC in the prediction model of tumor grade is worth discussing. From the decision tree analysis, there were six significant predictors of HG-BC: age, number of lesions, stalk, tumor diameter, tumor contact length, and basal width. Among the six risk factors, some may influence each other. Hence, the construction of the DT-model is an ideal method to predict HG-BC. The most obvious advantage of this study is that high-grade MIBC patients were excluded by applying a high VI-RADS score, making the DT-model established in patients with non-MIBC (VI-RADS ≤2). This not only provides a comprehensive interpretation of the clinical benefit of high VI-RADS but also narrows the target population scope of the HG-BC predictive model to patients with NMIBC, and improves its accuracy.Conclusion
The majority of patients with VI-RADS score ≥3 are HG-BCs. VI-RADS could be used as a grouping imaging biomarker for a pathological grading prediction model, which in combination with the DT-model for low VI-RADS (≤2) populations could provide a potential preoperative non-invasive method of predicting HG-BC.Acknowledgements
NoneReferences
1. Cambier S, Sylvester RJ, Collette L et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guerin. Eur Urol. 2016;69(1):60-9
2. Ravvaz K, Walz ME, Weissert JA, Downs TM. Predicting Nonmuscle Invasive Bladder Cancer Recurrence and Progression in a United States Population. J Urol. 2017;198(4):824-831
3. Babjuk M, Burger M, Capoun O et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75-94
4. Witjes JA, Bruins HM, Cathomas R et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. 2021;79(1):82-104
5. Panebianco V, Narumi Y, Altun E et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur Urol. 2018;74(3):294-306
6. Wang H, Luo C, Zhang F et al. Multiparametric MRI for Bladder Cancer: Validation of VI-RADS for the Detection of Detrusor Muscle Invasion. Radiology. 2019;291(3):668-674
7. Cao B, Li Q, Xu P et al. Preliminary Exploration of the Application of Vesical Imaging-Reporting and Data System (VI-RADS) in Post-treatment Patients With Bladder Cancer: A Prospective Single-Center Study. J Magn Reson Imaging. 2022;55(1):275-286
8. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70(1):106-119
Figures