4269
Early Detection of Pancreatic Cancer by Hyperpolarized Magnetic Resonance and pO2 Electron Paramagnetic Resonance Imaging1Cancer System Imaging, UT MD Anderson Cancer Center, Houston, TX, United States, 2UT MD Anderson Cancer Center UT Health Science Center Houston Graduate School of Biomedical Sciences, Houston, TX, United States, 3Clinical Cancer Prevention, UT MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Keywords: Cancer, Pancreas, Metabolism, Metabolic Imaging, Hyperpolarized MR (non-gas)
There is an unmet need for the early diagnosis of pancreatic cancer. Diagnosis is difficult due to the asymptomatic nature of pancreatic cancer. One way to detect the early stages is to monitor the altered metabolism in premalignant pancreatic lesions in vivo with hyperpolarized metabolic imaging. Here we demonstrate how genetically engineered mouse models were used to detect early stages of pancreatic cancer as we see an increase in the altered metabolism in the pancreatic cancer models compared to control mice. Simultaneously we observe changes in hypoxia levels in these models using electron paramagnetic resonance imaging.Introduction
Pancreatic cancer is one of the most aggressive types of cancers. It is difficult to detect due to its asymptomatic presentation at early stages. Therefore, there is an unmet need for non-invasive imaging markers that help identify the aggressive sub-type(s) in a pancreatic lesion at an early time point in pancreatic cancer. One of the most commonly used imaging biomarkers is the conversion of hyperpolarized pyruvate to lactate and alanine.1 It has been previously demonstrated that at early timepoints of pancreatic cancer the Warburg effect kicks in and promotes the conversion to lactate. At the same time, hypoxic conditions have been associated with pancreatic cancer progression and therapeutic resistance.2 With metabolic HP-MR imaging, the conversion will be monitored between different premalignant models at different timepoints. At the same time, the emerging electron paramagnetic resonance (EPR) imaging will be utilized for interrogating the hypoxia levels as these premalignant models progress to pancreatic cancer.Methods
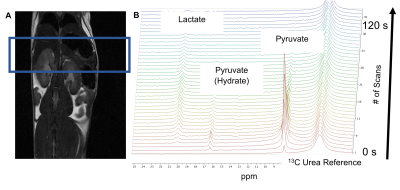
Hyperpolarized 1-13C Pyruvate MRS was employed to study the metabolic processes in tamoxifen inducible genetically engineered mouse (GEM) models (P48CreERT2;LSL-KrasG12D (iKC)) with pre-invasive pancreatic intraepithelial neoplasia (PanIN) precursor lesions, invasive pancreatic cancer model (P48CreERT2;LSL-KrasG12D; LSL-p53R172H (iKPC)) and control animals (P48CreERT2 (iC)) without pancreatic lesions. The dissolution DNP (HyperSense, Oxford Instruments) operating at 3T was utilized to hyperpolarize 1-13C pyruvate. The 13C magnetic resonance spectra of hyperpolarized 1-13C pyruvate were acquired at 7T Bruker MRI scanner.3 (Figure 1) These mice were imaged at different time points in their lifespan, before tamoxifen induction, 10-, 20-, and 30-weeks post induction. Simultaneously, EPR imaging data are collected at later timepoints, after pancreatic lesions have been observed.Results/Discussion
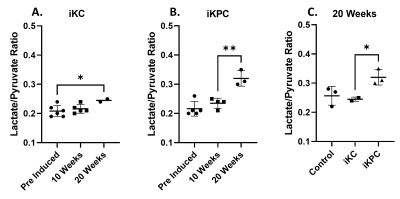
The alanine-to-lactate signal intensity ratio was found to decrease as the disease progressed from low-grade PanINs to high-grade PanINs. At the same time, the lactate-to-pyruvate ratio increased in the pancreatic cancer models compared to the control model. These results demonstrate that there are significant alterations of ALT and LDH activities during the transformation from early to advanced PanINs lesions. As for the aggressive iKPC mouse model, at the 20-week post induction imaging there was a significant increase of the lactate-to-pyruvate ratio (0.32) compared to the 10-week time point after induction (0.24). The 20-week iKPC time point ratio compared to the iKC and control mouse models was significantly higher, (0.32 compared to 0.25 and 0.26 respectively) indicating the invasive nature of the cancer. Even in the iKC model there is a slight increase of the lactate-to-pyruvate ratio at 20-weeks post induction (0.25) compared to both previous time points, pre-induction (0.21) and 10-week (0.22). All this data is shown in Figure 2. The aggressiveness of this models was also observed as none of the mice survived until the third timepoint at 30-weeks post induction. In the future, imaging at earlier timepoints or at smaller intervals would be helpful in the iKPC model, to observe the exact moment the cancer became invasive. With EPR imaging we observed increasing hypoxia levels with PanIN progression. We plan to implement some Artificial Intelligence (AI) components to our metabolic profiles to further predict pancreatic cancer at even earlier stages.4Conclusion
Findings from this HP-MR and EPR Imaging techniques can be potentially translated to the clinic for detection of pancreatic premalignant lesion in high-risk populations. With the future addition of AI, we hope to facilitate the translation to the clinic sooner.Acknowledgements
This research was funded in part by a grant from Pancreatic Cancer Action Network (PANCAN; 16-65-BHAT) (PKB, FM); NCI PREVENT (PKB, FM); Duncan Family Institute for Cancer Prevention and Risk Assessment Seed Funding; by grants from the US National Cancer Institute (U01 CA214263, U54 CA151668 and R21 CA185536, R01 CA218004; and 1P50 CA221707-01). This work also was supported by the National Institutes of Health/NCI Cancer Center Support Grant under award number P30 CA016672.References
1. Dutta, P., Pando, S. C., Mascaro, M., et al. Early Detection of Pancreatic Intraepithelial Neoplasias (PanINs) in Transgenic Mouse Model by Hyperpolarized 13C Metabolic Magnetic Resonance Spectroscopy. International Journal of Molecular Sciences. 2020; 21(10), 3722.
2. Yamasaki, A., Yanai, K. and Onishi, H. Hypoxia and pancreatic ductal adenocarcinoma. Cancer letters. 2020; 484, 9-15.
3. Pudakalakatti, S., Raj, P., Salzillo, T. C., Enriquez, J. S., et al. Metabolic Imaging Using Hyperpolarization for Assessment of Premalignancy. In Cancer Immunoprevention. 2022; 169-180. Humana, New York, NY.
4. Enriquez, J.S., Chu, Y., Pudakalakatti, S., et al. Hyperpolarized magnetic resonance and artificial intelligence: Frontiers of imaging in pancreatic cancer. JMIR medical informatics. 2021; 9(6), p.e26601.
Figures