4268
Metabolic Imaging of Pancreatic Ductal Adenocarcinoma
Saleem Yousf1, Kristine Glunde1,2, Dalton R. Brown1, Caitlin Tressler1, Michael G. Goggins3,4,5, and Zaver M. Bhujwalla1,2
1Division of Cancer Imaging Research, The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Departments of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Departments of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5Departments of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States
1Division of Cancer Imaging Research, The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Departments of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Departments of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5Departments of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Keywords: Cancer, Metabolism, PDAC
Metabolic imaging techniques such as mass spectrometry imaging (MSI) complement magnetic resonance spectroscopy and colocalize metabolite profiles with immunohistological evaluation of tissue sections. Here we have performed matrix-assisted laser desorption/ionization (MALDI)-MSI of normal pancreatic tissue, intraductal papillary mucinous neoplasm (IPMN), and pancreatic ductal adenocarcinoma (PDAC) to characterize the spatial distribution of PDAC metabolites in tissue sections. Our preliminary data with MALDI-MSI identifies significantly increased taurine and significantly decreased creatine, both of which can also be detected with 1H MRS, in PDAC compared to normal tissue. The spatial heterogeneity of metabolite distribution in PDAC tissue can be related to immunohistochemical evaluation.Introduction
Metabolism is the old ‘new’ frontier in cancer, with metabolites being associated with multiple functional outcomes including immune suppression. Mass spectrometry imaging (MSI) can identify metabolites that can be developed as biomarkers for 1H MRS, and provide insights into the ‘cold’ immune microenvironments of PDAC that make it poorly responsive to cancer immunotherapy. Here we performed MALDI-MSI on human normal pancreatic tissue, IPMN and PDAC to characterize alterations in metabolism in PDAC compared to normal pancreatic tissue and IPMN.Methods
Studies were performed with deidentified normal (n=5), IPMN (n=5) and PDAC (n=5) tissues. For MALDI mass spectrometry imaging, tissue samples were cryo-sectioned at 10-μm slice thickness, and thaw-mounted onto conductive indium tin oxide-coated glass slides (Delta Technologies) and stored at -80 °C prior to analysis. Tissue sections were vacuum-dried in a desiccator at room temperature for 10 min prior to matrix application. 1,5-diaminonaphthalene (10 mg/ml) in 70% acetonitrile with 0.2% trifluoroacetic acid was applied using an HTX M5 sprayer (HTX Technologies). MALDI Imaging was performed on a Bruker RapifleX MALDI TOF/TOF instrument both in positive and negative ion modes. Both positive and negative ion modes were acquired from m/z 39-1000, with 200 micron raster size and 200 laser shots per pixel. MALDI images were generated using flexImaging and analysis was performed with SCiLS Lab software. Candidate metabolites were identified against the Human Metabolome Database (http://www.hmdb.ca/) and the Lipid Maps Database (www.lipidmaps.org). Matches were confirmed with the MS/MS data acquired on the Bruker TimsTOF fleX MALDI-2. After MSI experiments, all slides were washed and stained with hematoxylin-eosin (H&E) for histological referencing.Results
We identified several metabolites altered in PDAC compared to normal tissue and IPMN. Of these, taurine and creatine are two metabolites that are detectable by 1H MRS in vivo that could be investigated as biomarkers of PDAC. MS images of the taurine (m/z 123.979) maps in tissue sections are presented in Figure 1 and summarized in Figure 2. Taurine levels were almost three-fold in PDAC compared to normal pancreatic tissue. MS images of creatine (m/z 130.038) in tissue sections are presented in Figure 3 and summarized in Figure 4. A significant decrease in creatine was observed in PDAC compared to normal tissue.Discussion
A previous study has reported an increase of taurine in early pancreatic cancer using HR-MAS MRS1. Taurine has also been found to promote immune suppressive Tregs2 in asthma. MSI identified a heterogenous distribution of taurine in tumor sections. We are currently performing immunohistochemistry to relate the taurine distribution to immune or other cells in tumor sections. Increased taurine and decreased creatine can be developed as biomarkers of PDAC for in vivo 1H MRS/I.Acknowledgements
Support from NIH R35 CA209960 and the Emerson Collective is gratefully acknowledged. We thank Dr. Krishnamachary for his assistance.References
1. Alan S Wang, Alessia Lodi, Lee B Rivera, Jose L Izquierdo-Garcia, Matthew A Firpo, Sean J Mulvihill, Margaret A Tempero, Gabriele Bergers, Sabrina M Ronen. HR-MAS MRS of the pancreas reveals reduced lipid and elevated lactate and taurine associated with early pancreatic cancer. NMR Biomed. 2014 Nov;27(11):1361-70. doi: 10.1002/nbm.3198. Epub 2014 Sep 9.
2. Jing Zhou, Yi Lu, Wei Wu, Yunhai Feng. Taurine promotes the production of CD4+CD25+FOXP3+ Treg cells through regulating IL-35/STAT1 pathway in a mouse allergic rhinitis model. Asthma Clin Immunol. 2021 Jun 19;17(1):59.
Figures
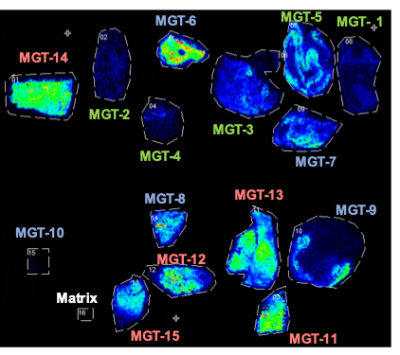
Spatial distribution of taurine (m/z 123.979) in normal pancreatic tissue (MGT1-5), IPMN (MGT6-10), and PDAC (MGT 11-15). A clear increase of taurine is evident in PDAC.
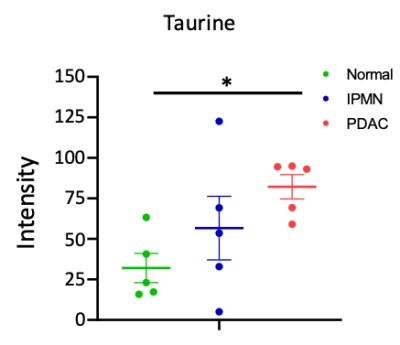
Quantification of taurine intensity in each sample. Taurine levels in PDAC increased by three-fold compared to normal pancreatic tissue. *P<0.05.
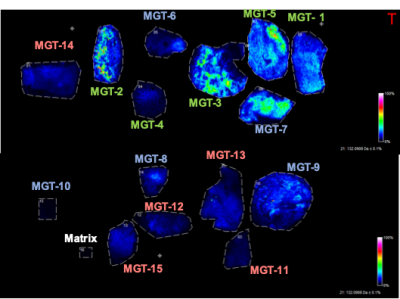
Spatial distribution of creatine (m/z 130.038) in normal pancreatic tissue (MGT1-5), IPMN (MGT6-10), and PDAC (MGT 11-15). A clear decrease of creatine is evident in PDAC
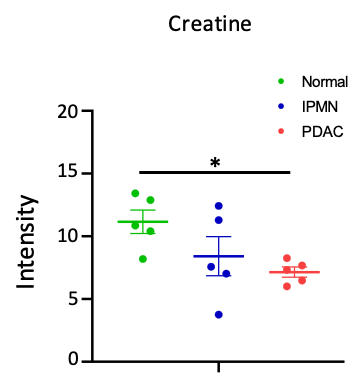
Quantification of creatine intensity in each sample. Creatine levels in PDAC decreased significantly compared to normal pancreatic tissue. *P<0.05.
DOI: https://doi.org/10.58530/2023/4268