4262
A 3T Coil Array for Improved Quantitative Susceptibility Mapping at Multiple Orientations in ex vivo Primate Brains1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Giessen, Germany, 2Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Felix Bloch Institute for Solid State Physics, Leipzig University, Felix Bloch Institute for Solid State Physics, Leipzig, Germany
Synopsis
Keywords: RF Arrays & Systems, Quantitative Susceptibility mapping
Quantitative susceptibility mapping (QSM) with multiple object orientations is typically limited by an appropriate examination time that is appropriate for human subjects and the small range of possible rotation angles within an MRI head coil. Ex vivo QSM can overcome these limitations supporting long acquisition times and the ability to rotate the ex vivo specimen to any position. Therefore, a 3T 26-channel ex-vivo brain array coil was developed for fixed chimpanzee brains. The coil was characterized with bench and image metrics and provides increased reception sensitivity, making it well-suited for high-resolution QSM ex vivo MRI studies.INTRODUCTION
The recent discovery of magnetic susceptibility anisotropy in white matter1-3 has sparked a great deal of interest in using susceptibility properties as a potential complementary method for uncovering brain microstructure, including axonal characteristics4-6. However, obtaining accurate susceptibility maps constitutes an ill-posed problem, since the kernel that inversely couples the field to its susceptibility distribution contains zeros. A viable technique to obtain the missing data of the susceptibility is to reorient the sample within the static magic field and re-acquire the MRI signal7.Given the maximum examination time appropriate for human subjects, it is impractical to repeatedly acquire data from the brain at different orientations. Ex-vivo brain scans of fixed tissue, however, can overcome this limitation by providing “unlimited” acquisition time. Moreover, fixed tissue samples allow a high degree of freedom for scanning at various orientations, which would not be physically possible with human subjects. The aim of this study was the design and construction of a dedicated 26-channel ex vivo brain array coil, suitable for fixed chimpanzee brains, which can be freely oriented within the coil former. The coil was validated through initial bench-level and imaging-level metrics.
METHODS
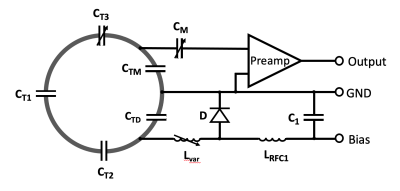
The coil former has a spherical shape to allow any orientations of choice for the brain sample. It can accommodate whole ex vivo chimpanzee brains up to longitudinal size of 145 mm. The layout of the overlapped coil elements was derived from a hexagonal and pentagonal tiling pattern to accommodate the 3D circuitry of the element arrangement.7 The entire coil loop pattern was printed onto the coil former together with standoffs to mount the coil adjacent preamplifiers. The average loop diameter was 80 mm. All helmet parts including its covers were printed in polycarbonate plastic using a rapid prototyping 3D printer (Fortus 360, Stratasys Ltd., Eden Prairie, MN, USA). The coil housing was split into two segments. It comprises 13 elements on each side, which were made of 1.5mm wire loops. For sufficient decoupling between the individual elements, all neighboring loops were critically overlapped. The overlap between the bottom and top housing elements was mechanically achieved by incorporating an overlapping rim structure. Next-nearest neighboring coil elements were decoupled using preamplifier decoupling 8, by transforming the preamplifier’s input impedance to a high series impedance within the loop.Bench measurements verified the element’s tuning, matching, active detuning, nearest-neighbor coupling and preamplifier decoupling. Additionally, QU/QL-ratio was obtained with a coil element under test within the populated but detuned array assembly. Pairs of coils were attached to a twin preamplifier and low-noise converters (Siemens, Healthineers, Erlangen, Germany).
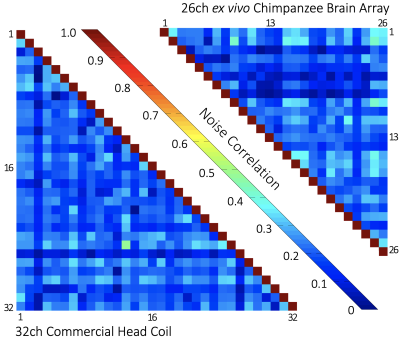
A fixed brain of an animal that had died from natural causes in a zoo was immersed in Fomblin and positioned in a 3D-printed container adapted to its individual brain anatomy6. This container was then mounted in a spherical holder and placed inside the constructed coil. Initial imaging was carried out on a 3T CONNECTOM Scanner (Siemens Healthineers, Erlangen, Germany). The array’s noise correlation and the signal-to-noise ratio (SNR) were calculated from complex 3D single-echo GRE data (0.8 mm isotropic nominal resolution, TE at 25ms, without GRAPPA acceleration or Partial Fourier) and compared to the results obtained with a standard 32-channel whole-head coil.
RESULTS
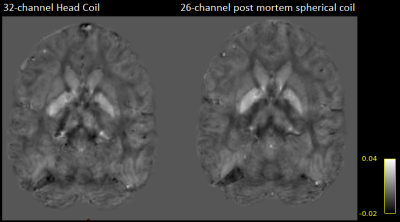
The ex vivo primate brain coil showed nearest-neighbor element decoupling of –12 dB and preamplifier decoupling of –21 dB. The coupling between non-adjacent coils ranged from –11 dB to –31 dB. The QU/QL-ratio was 229/49=4.7. The SNR increased by 2.7-fold and 1.4-fold in the cortex and brain center, respectively, when compared to the standard 32-channel head array. The noise correlation between the channels ranged from 0.02% to 41% (avg. ~18%). Initial susceptibility comparisons showed improved contrast details.DISCUSSION
Using standard head coils for small-sized ex vivo brain studies render brain imaging suboptimal. These coils have a large distance between the sample and coil elements and are not able to entirely enclose the ex vivobrain, due to the commonly employed in vivo head coil topology, allowing the patient to be placed inside the coil. The customized 26-channel ex vivo primate brain coil, addresses this limitation by providing entirely surrounding coil elements to the close proximity of the ex vivo brain, thus providing increased reception sensitivity. This is particular important for enabling methods like Susceptibility Tensor Imaging (STI) as well as High Angular Resolution Susceptibility Imaging (HARSI) for ODFs estimation6, where obtained phase maps with consistent sensitivity from all positioned orientations of the sample are highly desirable. The constructed coil provides both the mechanical ability for rotating the ex vivo brain and increased SNR by a factor of 2 on average over the whole brain volume in comparison to a 32-channel human head coil.CONCLUSION
A 26-channel ex vivo coil array was constructed for a close fit to whole chimpanzee brains, with small receive elements distributed over the entire volume. The brain sample can be rotated in any position, while maintaining high SNR of the fixed brain. This would be well-suited for methodologies requiring high angular orientation sampling, like STI and HARSI.Acknowledgements
No acknowledgement found.References
[1] He X, et al. Biophysical mechanisms of phase contrast in gradient echo MRI. Proc. Natl. Acad. Sci. U. S. A. (2009); 106(32): 13558–13563.
[2] Lee J, Shmueli K, Fukunaga M, van Gelderen P, Merkle H, Silva AC, Duyn JH. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proc. Natl. Acad. Sci. U. S. A. (2010); 107(11): 5130–5135.
[3] Liu C. Susceptibility tensor imaging. Magn. Reson. Med. (2010) 63(6): 1471–1477.
[4] Liu C, et. al. 3D fiber tractography with susceptibility tensor imaging. NeuroImage 2012; 59(2): 1290–1298.
[5] Gkotsoulias DG, et al. High angular resolution susceptibility and diffusion imaging in post mortem chimpanzee brain: Tensor characteristics and similarities. Proceedings of the ISMRM, 31st Annual Meeting, 2022, # 3966.
[6] Gkotsoulias et al., High Angular Resolution Susceptibility Imaging and Estimation of Fiber Orientation Distribution Functions in Primate Brain, bioRxiv 2022.10.23.513390; doi: https://doi.org/10.1101/2022.10.23.513390.
[7] Wiggins GC, et. al. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. (2006) 56(1):216-23. [8] Roemer PB, et al. The NMR phased array. Magn. Reson. Med. (1990) 16(2):192-225.
Figures