4259
In-bore Reclinable Array for Pediatric Spine Imaging1Electrical and Computer Engineering, McGill University, Montréal, QC, Canada, 2Ophthalmology, McGill University, Montréal, QC, Canada
Synopsis
Keywords: RF Arrays & Systems, RF Arrays & Systems
We constructed and tested a reclinable MR compatible chair equipped with an 8-channel spine array coil for 3T. The coil used a combination of overlap and transformer decoupling to maximize spatial resolution without modifying individual coil size. The signal to noise ratio and noise correlation matrices were used to compare the performance of the array to a 32-channel commercial array of the same type. The SNR variations were tested with respect to the inclination of the chair.
Introduction
Pediatric imaging presents unique sets of challenges ranging from anatomical, developmental, physiological to behavioral. Many of those, can be mitigated by moving to higher MRI fields and designing better fitted coils resulting in higher image quality and spatial resolution1. However, due to the age of the patients, certain tasks remain challenging such as holding their breath or sitting still long enough during a scanning session2. As a result, current protocols involve sedation/general anesthesia or behavioral training in mock scanners. Despite their effectiveness, these solutions carry potential risks and/or require trained personnel incurring additional costs and wait times before the scan. In recent years, audio-visual integration boosted the number of successful pediatric scans3. With that in mind, one could envision a self-contained capsule capable of accommodating the patient along with different entertainment systems. Given the dimensions of most MRI bores, inclination of the capsule would be required. To investigate the effect of inclination on the quality of the images, we designed and constructed a MR compatible reclinable chair equipped with a custom 8-channel spine array to be used with the Prisma scanner.Methods
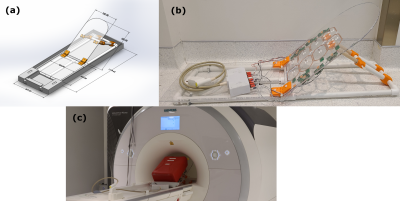
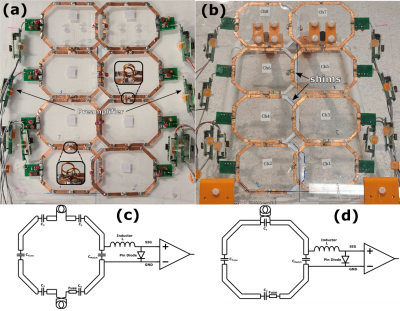
Due to the size of the bore, the chair, presented in Figure 1(a), was limited to children up to 5 years old. Its dimensions were based on the child anthropometry study conducted by the transportation research institute of the University of Michigan4. The chair was fully non magnetic, constructed with a combination of off the shelf and 3D printed parts. Additionally, it was equipped with nylon screws allowing adjustment of the back inclination.The 8-channel array was attached to the backside of the chair as seen in Figure 1(b). Each coil was 120mm x 80mm with chamfered edges constructed using 6mm width copper foil tape. The loops were segmented using 4 or 6 capacitors with each segment having a length less than λ/20. Overlap decoupling was used for the horizontal alignment with 3D printed inserts used to avoid shorting the coils as shown in Figure 2(b). For the vertical alignment, transformer decoupling was used to maximize spatial resolution without increasing the size of the coils5. This was achieved by using 2-turn mini-coils of 8mm diameter constructed with 11AWG copper wire as seen in Figure 2(a). Two types of coils were developed depending on the number of mini loops used as shown in Figure 2(c) and 2(d). Preamplifier decoupling was achieved by using a coax cable of 30mm length. All elements were tuned to 123.25Mhz and matched, when loaded, to 200Ω using a custom pi-network connected to a 2-port VNA. As a load, a custom 20L phantom was used to mimic the human torso.
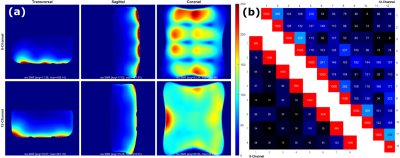
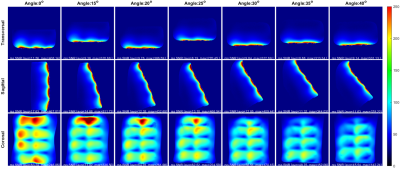
The imaging was conducted on a 3T Prisma scanner (Siemens Healthineers, Erlangen, Germany). Signal-to-noise-ratio(SNR) images and noise correlation matrices were generated using a PD-weighted FLASH sequence (known as SNRmap6) using the following parameters: TE=6 ms, TR=30 ms, FoV=400mm×400mm, BW=390 Hz/Pixel, and FA=10o. The results were compared to a 32-channel spine array with only 12-channel enabled. Axial, sagittal, and coronal slices were imaged for each inclination starting from 15⁰ to 40⁰ with 5 degrees increments.
Results
The fused elements of the array averaged a Q unloaded to Q loaded ratio of ~75/15=5. The double probe S12 measurements ranged from -21.6dB to -18.2dB for the coil elements. The isolation of the active detune averaged around 23.5 dB which can be improved later on by adding a passive detune circuit. On the other hand, the preamplifier decoupling was around 17.6dB. Figure 3 shows the SNR and noise covariance comparison between the custom array and the 32-channel array without any inclination. In most cases, the custom coil outperforms the commercial one. In fact, the sagittal and coronal slices show a 2-fold SNR increase toward the surface of phantom and a 1.2 to 1.4 increase toward the middle of the sample. The axial slice shows similar results with SNR degradation toward the edges of the phantom. For the noise covariance matrices, the average correlation noise was 5.2% for the custom array which is significantly lower than the 12.1% of the 32-channel array. Figure 4 shows that whenever the inclination angle is increased the overall SNR of the coil is attenuated. In fact, the SNR loss between 0o and 40o was estimated to be around 21% in the transverse slice, 34% in the sagittal and 45% in the coronal slice. Additionally, a slight geometric distortion is also seen toward the top of the image when the angle exceeds 25o. However, despite that, the average SNR remained higher than 20 for depth up to 30mm for all angles.Conclusion
This system allows patients scanning under different inclination inside the MRI bore. Despite the SNR attenuations, the system proved that this type of imaging is still feasible opening the door to more creative imaging methods.Acknowledgements
We thank William Mathieu for his advice and assistance during this project.References
1. C. Dagia and M. Ditchfield, “3T MRI in paediatrics: Challenges and clinical applications,” vol. 68, pp. 309–319, Nov. 2008.
2. Y. Arlachov and R. H. Ganatra, “Sedation/anaesthesia in paediatric radiology,” vol. 85, pp. e1018–e1031, Nov. 2012.
3. C. Lemaire, G. R. Moran, and H. Swan, “Impact of audio/visual systems on pediatric sedation in magnetic resonance imaging,” Journal of Magnetic Resonance Imaging, vol. 30, pp. 649–655, Sept. 2009.
4. K. Weber, R. J. Lehman, and L. W. Schneider, “Child anthropometry for restraint system design,” Deep Blue Repositories, University of Michigan, Jan 1985. [Online]. Available: https://deepblue.lib.umich.edu/handle/2027.42/172.
5. N. I. Avdievich, “Transceiver-phased arrays for human brain studies at 7T,” Applied Magnetic Resonance, vol. 41, pp. 483–506, Oct. 2011.
6. Polimeni, J.R. & Wald, L.L. Documentation for IceMGHCoilArrayReconUtil software, Harvard Medical School, 2009.
Figures