4241
Towards high sensitivity 13C MRS of Human Brain Glycogen at 7T
Eulalia Serés Roig1
1Laboratory of Functional and Metabolic Imaging (LIFMET), Institute of Physics (IPHYS), School of Basic Sciences (SB), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
1Laboratory of Functional and Metabolic Imaging (LIFMET), Institute of Physics (IPHYS), School of Basic Sciences (SB), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
Keywords: Phantoms, Non-Proton
Largely neglected due to its low concentration, yet brain glycogen plays an active role in brain energy metabolism and may be involved in a variety of brain diseases. The application of 13C MRS along with 13C-glucose intravenous infusion is to date the forefront method for investigating brain glycogen metabolism in vivo. Notably, the C1-resonances of glycogen and glucose have been detected non-invasively in the conscious human brain by 13C MRS at 4T after 13C-glucose infusion labelled at the C1-carbon. In this study, we explore the potential of 13C MRS at 7T with broadband 1H-decoupling towards measuring glycogen and glucose C1-resonances.Introduction
Although accepted as the main energy storage in the central nervous system, the role of glycogen in the conscious human brain is largely unknown. Localized carbon-13 magnetic resonance spectroscopy (13C MRS) allows the non-invasive detection of human brain glycogen in vivo, which, due to the low concentration of glycogen in the brain, is typically done via 13C-glucose intravenous infusion labelled at the C1-carbon to enhance the 13C sensitivity while giving rise to three C1-resonances: glycogen, glucose-β, and glucose-α1, 2. To further improve both sensitivity and spectral resolution, the use of ultra-high magnetic field (i.e., ≥ 7T) has the clear advantage of separating the resonances to better facilitate their quantification. In this context, the aim of this study was to explore the potential of 13C MRS at 7T in conjunction with broadband 1H-decoupling while adjusting the decoupling scheme parameters towards optimal sensitivity and simultaneous detection of glycogen and glucose C1-resonances.Methods
In vitro 1H-decoupled 13C MRS measurements of glycogen and glucose C1-resonances were performed on a 7T human scanner (Siemens Erlangen/Germany) using a home-built 13C-linear/1H-quadrature RF surface coil. A pulse-acquire sequence for localized 13C MRS using broadband 1H-decoupling during 13C signal acquisition was developed using the WALTZ-16 scheme3 (Figure 1). All in vitro measurements were performed using a two-compartment phantom containing 1) 800mM natural abundance of glycogen and 2) 8mM of glucose-C1 labelled, while all spectra were acquired using uniform adiabatic 13C-excitation (2ms)4 by placing the carrier frequency at the glucose-β resonance (96.6ppm). The performance of the 1H-decoupling scheme was investigated by increasing successively the number of WALTZ cycles from 1 to 8 (i.e., 4 WALTZ cycles corresponds to the WALTZ-16 scheme), while adjusting in each case the decoupling duration accordingly to the FID (~96ms). To that purpose, the duration of the main WALTZ-cycle 90°-pulse was successively decreased from 4ms (1 WALTZ cycle) to 0.5ms (8 WALTZ cycles), such that in all cases the decoupling duration matched that of the FID (~96ms). In addition, the spin-lattice (13C-T1) and spin-spin (13C-T2) relaxations times of glycogen and glucose C1-resonances were measured in vitro using optimized adiabatic an inversion-recovery and Hahn spin-echo5 sequences, over a range of inversion times (TI) and echo times (TE), respectively.Results and Discussion
In vitro 1H-decoupled 13C MRS at 7T revealed three well resolved C1-resonances including glycogen (100.5ppm), glucose-β (96.6ppm) and glucose-α (92.8ppm), as by comparing spectra without and with broadband 1H-decoupling using the WALTZ-16 scheme (Figure 2 A left). By successively increasing the number of WALTZ cycles, the 13C signal intensities of glucose-β and glucose-α increased or decreased depending on whether an odd or an even number of WALTZ cycles was applied, respectively (Figure 2 A, B), and this could be attributed to the fact that the 1H-spin ended up either down (Figure 2 A/ green-arrows) or up (Figure 2 A/ red-arrows), respectively. In contrast, no such effect was observed for glycogen in which the 13C signal intensity remained fairly constant (Figure 2 A, B). The slight decrease of the glucose-β slope (Figure 2 B) could be attributed either to the presence of sidebands (Figure 2 A bottom/ ocher-arrows) and/or to the proximity of glucose-β 1H-resonance to that of water (Figure 2 C). In contrast, a slight increase on the glycogen and glucose-α slopes was observed upon increasing the number of WALTZ cycles (Figure 2 B). In fact, despite the observed sidebands in the glucose-β resonance as of 5 WALTZ cycles applied, an improved decoupling efficiency is expected by increasing successively the number of WALTZ cycles beyond 8, as suggested by the slight increase of the slopes of glycogen and glucose-α from 1 to 8 WALTZ cycles. Note that, given the FID duration of the 13C signal of glycogen and glucose C1-resonances (~96ms) and the 1H chemical shift range comprising the glycogen and glucose H1-resonances (~0.9ppm, i.e., ~270Hz), a minimum of 3 WALTZ cycles were needed to achieve a decoupling bandwidth broad enough to fully cover glycogen and glucose H1-resonances, thus, to fully decouple the glycogen and glucose C1-resonances (Figure 2 A, C). Besides, the 13C-T1 and 13C-T2 of glycogen C1 in vitro (Figure 3 B, C) were found in close agreement with what is expected at 7T compared to adjacent field strengths6, while the small differences of T2 compared with the literature6 may be due to differences in temperature. The 13C-T1 of glucose-α and glucose-β in vitro were found to be rather similar to each other (Figure 3 B), while their 13C-T2 were slightly disparate (Figure 3 C).Conclusion
In this study, the simultaneous detection and sensitivity of glycogen and glucose C1-resonances were evaluated by 1H-decoupled 13C MRS in vitro at 7T while adjusting the decoupling parameters of the WALTZ-16 scheme. A clear improved spectral resolution in the detection of glycogen and glucose C1-resonances at 7T was observed compared to 4T1,2. The sensitivity of glucose C1-resonances increased or decreased depending on whether an odd or an even number of WALTZ cycles was applied, respectively, while this effect was not observed for glycogen C1-resonance. Overall, the use of 3 versus 4 WALTZ cycles may be advantageous in terms of optimal 13C sensitivity and low 1H-power towards measuring human brain glycogen in vivo at 7T.Acknowledgements
This study was supported by Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL and the Leenaards and Jeantet Foundations.References
1. Gruetter R. JNR.2003;74(2):179-83
2. Oz G. NInt.2003;43:323-9
3. Shaka AJ.JMR.1983;52:335-338
4. Serés Roig E. NMR Biomed. 2019
5. Hahn EL Phys. Rev. 1950;80,580
6. Sillerud LO. Bioch1983;22,1087-1094
Figures
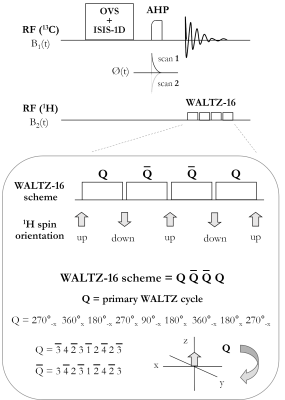
Figure 1: Schematic diagram of the pulse-acquire sequence for 1H-decoupled localized 13C MRS at 7T. The sequence consists of 13C spatial localization using the OVS and ISIS-1D schemes, followed by uniform 13C adiabatic excitation using two AHP pulses with inverted phases applied in alternate scans, and broadband 1H decoupling using the WALTZ-16 scheme during 13C signal acquisition. Each WALTZ cycle (Q) performs an inversion of the 1H-spin, the latter resulting oriented down or up in the z direction depending on whether an odd or an even number of WALTZ cycles is applied, respectively.
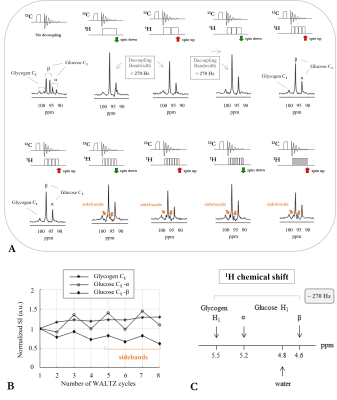
Figure 2: (A) Serie of 13C MR spectra acquired in vitro at 7T on a two-compartment phantom containing solutions of 800mM natural abundance glycogen (inner) and 8mM glucose C1-labelled (outer). Spectra were acquired without (top left spectrum) and with 1H-decoupling by increasing successively the number of WALTZ cycles from 1 to 8. (B) Graphic showing the normalized 13C signal intensities of glycogen and glucose C1-resonances (from spectra in (A)) as a function of the applied number of WALTZ cycles. (C) Schematic diagram of the 1H chemical shifts of glycogen and glucose H1-resonances.
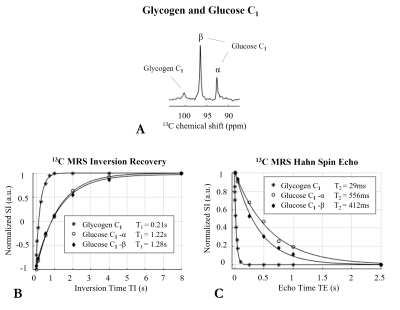
Figure 3: (A) 1H-decoupled 13C MR spectrum acquired in vitro at 7T on a two-compartment phantom containing solutions of 800mM natural abundance glycogen (inner) and 8mM glucose C1-labelled (outer). Inversion recovery (B) and spin echo (C) fitting curves of 13C signal intensities of glycogen and glucose C1-resonances acquired in vitro using optimized adiabatic an inversion-recovery and Hahn spin-echo sequences, over a range of inversion times (TI) and echo times (TE), respectively. T1 and T2 were determined using three-parameter and single-exponential fitting, respectively.
DOI: https://doi.org/10.58530/2023/4241