4230
Predictive Value of Intrapixel Incoherent motion (IVIM) Imaging For Prognostic Factors in Breast Cancer1The Affiliated Hospital of Southwest Medical University, LUZHOU, China, 2MR Scientific Marketing, Siemens Healthineers Ltd, Shanghai, China
Synopsis
Keywords: High-Field MRI, Diffusion/other diffusion imaging techniques, Intravoxel Incoherent MotionImaging
The objective of this study was to estimate the true diffusion and motion of molecules in the capillary network using the VIM biexponential model without injection of contrast media, three parameters (d value, d * value and F value) were obtained to evaluate the expression of prognostic molecules (ER, PR, Her-2, Ki-67) in breast cancer, which can guide the treatment of breast cancer more precisely.
Introduction
Breast cancer is the most common heterogeneous cancer in women worldwide. The expression of prognostic molecules in breast cancer (ER,PR,HER-2,Ki-67) has been observed to be closely related to disease outcome, treatment modality, and patient response to treatment, helping to guide more precise treatment and possibly predicting recurrence. The ADC value obtained by the traditional single-index diffusion model contains two kinds of information: microcirculation perfusion and tissue water molecule diffusion. As a non-enhanced imaging method, IVIM can display the physiological and pathological states of tissues without contrast agent injection. The IVIM biexponential model can also be used to estimate the three parameters (D value, D* value, f value) of the real diffusion and motion of molecules in the capillary network. The D value represents the true diffusion reflected by pure molecular diffusion, excluding the reflection of microcirculation in the intercellular space. D* value is the pseudo-diffusion coefficient of perfusion-related diffusion or incoherent microcirculation, and f value is the proportion of perfusion affected by microcirculation in tissue capillaries. This study intends to evaluate the prognostic value of several parameters (D value, D*, f value) obtained by IVIM magnetic resonance imaging in breast cancer.Method
A retrospective analysis was performed on the patients who underwent breast MRI examination and they were diagnosed as breast cancer by final surgery or biopsy in our hospital from July 2020 to May 2021. The patient's lesion was unilateral and the short diameter was greater than 1 cm, and the pathological data was complete. Patients with poor image quality that are not conducive to delineation were excluded. The final study included 171 breast cancer patients who underwent preoperative magnetic resonance imaging (MRI) on a 3T system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany), including morphological T2W imaging, T2W fat suppression imaging, IVIM imaging and T1-3D imaging. HAST sequence was used for morphological T2W images, TIRM sequence was used for T2W lipid-compression images, and T1-VIBE sequence was used for T1-3D images. IVIM imaging parameters were as follows :TR/TE = 5400/63 b values=0, 50, 100, 150, 200, 250, 300, 400, 600, 800sec/mm2; View = 340 x 170 mm; Voxel size = 1×1×4.0 mm3; 34 pieces; Parallel imaging factor = 2, acquisition time = 5 mins.The status of ER, PR, HER-2 gene amplification and the Ki-67 labelling index were assessed by immunohistochemistry. Institute guidelines. Positive ER and PR status was determined when immunostaining was positive in ≥ 1% of the cells. HER-2 overexpression were defined by fluorescence in situ hybridization(FISH) or with a pathology result of 3 (+), Ki67>14% was defined as positive.
According to the post-processing software of Siemens, the relevant parameter map was obtained, and two experienced doctors used the double-blind method to delineate the parameter values of interest at the maximum level. ER positive USES the independent t test evaluation (a) (n =103) and ER negative (n =68), and (b) PR positive (n =123) and PR negative (n =48), (c) PR positive (n =72) and PR negative (n =99), (d) K - i67 positive (n =137) and K - i67 negative (n =34) between d, d * f value difference. The diagnostic performance of T2 and IVIM parameters was determined by receiver operating characteristic (ROC) analysis. P<0.05 was considered statistically significant.
Result
The value of D and D* showed special significance. The values of D and D* in ER positive group were significantly lower than those in ER negative group (P = 0.001 and 0.007, respectively), and the value of D in PR positive group was significantly lower than that in negative group (P = 0.009). No significant difference between other parameters and prognostic factors was found for the time being (P > 0.05).ROC curve analysis shows that AUC values of D-value distinguishing ER and PR state are 0.665 and 0.681 respectively, and AUC value of D* distinguishing ER state is 0.621.Conclusion
IVIM-related parameters can predict the molecular status of breast cancer prognosis, and provide some basis for the determination of clinical treatment plan of breast cancer.The purpose of this study is to collect 171 breast cancer patients confirmed by IVIM examination and pathology, and compare the diagnostic values of different parameters in IVIM imaging to predict the prognostic molecular state of breast lesions. The IVIM related parameters of slow diffusion coefficient (D), fast diffusion coefficient (D*) and perfusion fraction (F) were measured, and the parameter values of different ER, PR, HER-2 and KI-67 states were evaluated and analyzed by ROC. Divided into (a) (n = 103) and er negative (n = 68), and (b) PR positive (n = 123) and PR negative (n = 48), (c) PR positive (n = 72) and PR negative (n = 99), (d) K - i67 positive (n =137) and K - i67 negative (n =34) 。 The results show that D value can distinguish ER and PR states, and D* can distinguish ER states (p <0.05), and the positive group is lower than the negative group (p <0.05), which is helpful to evaluate the prognosis molecular state of breast cancer and has certain guiding significance for the treatment of breast cancer.
Acknowledgements
This article was written under the guidance and care of Dr. Chen Jing. Dr. Chen Jing is knowledgeable, has a solid theoretical foundation, a rigorous academic attitude, and has always been at the forefront of academic research, in the paper research and writing process, many times to me to review the revision, and put forward a lot of good suggestions, so that my paper continues to improve, thanks to the Department of Leadership colleagues, during my thesis research for my care and help, is your encouragement to me more confident.
References
[1] Coates AS, Winer EP, Goldhirsch A, et al. . Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46.http://dx.doi.org/10.1093/annonc/mdv221
[2] Chen F, Chen P, Hamid Muhammed H, Zhang J. Intravoxel Incoherent Motion Diffusion for Identification of Breast Malignant and Benign Tumors Using Chemometrics. Biomed Res Int. 2017;2017:3845409. http://dx.doi.org/10.1155/2017/3845409
[3] Cho GY, Moy L, Kim SG, et al. Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur Radiol. 2016;26(8):2547‐2558.http://dx.doi.org/10.1007/s00330-015-4087-3
[4] Ma D, Lu F, Zou X, et al. Intravoxel incoherent motion diffusion-weighted imaging as an adjunct to dynamic contrast-enhanced MRI to improve accuracy of the differential diagnosis of benign and malignant breast lesions. Magn Reson Imaging. 2017;36:175‐179.http://dx.doi.org/10.1016/j.mri.2016.10.005
[5] Iima M, Yano K, Kataoka M, et al. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest Radiol. 2015;50(4):205‐211.http://dx.doi.org/10.1097/RLI.0000000000000094
[6] Lee YJ, Kim SH, Kang BJ, et al. Intravoxel incoherent motion (IVIM)-derived parameters in diffusion-weighted MRI: Associations with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging. 2017;45(5):1394‐1406.http://dx.doi.org/10.1002/jmri.25514
[7] Webber C, Gospodarowicz M, Sobin LH, Wittekind C, Greene FL, Mason MD, Compton C, Brierley J, Groome PA. Improving the TNM classification: findings from a 10-year continuous literature review. Int J Cancer. 2014 Jul 15;135(2):371-8.http://dx.doi.org/10.1002/ijc.28683
[8] Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 18.274.http://dx.doi.org/10.1093/annonc/mdz235
[9] Liu Y, Wang X, Cui Y, Jiang Y, Yu L, Liu M, et al. . Comparative Study of Monoexponential, Intravoxel Incoherent Motion, Kurtosis, and IVIM-Kurtosis Models for the Diagnosis and Aggressiveness Assessment of Prostate Cancer. Front Oncol (2020) 10:1763.http://dx.doi.org/10.3389/fonc.2020.01763
[10] Chen J, Liu S, Tang Y, Zhang X, Cao M, Xiao Z, et al. . Diagnostic Performance of Diffusion MRI for Pancreatic Ductal Adenocarcinoma Characterisation: A Meta-Analysis. Eur J Radiol (2021) 139:109672.http://dx.doi.org/10.1016/j.ejrad.2021.109672
[11]Lee SH, Shin HJ, Moon WK. Diffusion-Weighted Magnetic Resonance Imaging of the Breast: Standardization of Image Acquisition and Interpretation. Korean J Radiol. 2021 Jan;22(1):9-22.http://dx.doi.org/10.3348/kjr.2020.0093
[12] Mann RM, Cho N, Moy L. Breast MRI: State of the Art. Radiology. 2019 Sep;292(3):520-536.http://dx.doi.org/10.1148/radiol.2019182947
[13] He N, Li Z, Li X, et al. Intravoxel Incoherent Motion Diffusion-Weighted Imaging Used to Detect Prostate Cancer and Stratify Tumor Grade: A Meta-Analysis. Front Oncol. 2020;10:1623. http://dx.doi.org/10.3389/fonc.2020.01623
[14] Kakite S, Dyvorne H, Besa C, et al. Hepatocellular carcinoma: short-term reproducibility of apparent diffusion coefficient and intravoxel incoherent motion parameters at 3.0T. J Magn Reson Imaging. 2015;41(1):149-156. http://dx.doi.org/10.1002/jmri.24538
[15] Cui Y, Li C, Liu Y, et al. Differentiation of prostate cancer and benign prostatic hyperplasia: comparisons of the histogram analysis of intravoxel incoherent motion and monoexponential model with in-bore MR-guided biopsy as pathological reference. Abdom Radiol (NY). 2020;45(10):3265-3277. http://dx.doi.org/10.1007/s00261-019-02227-5
[16] orres-de la Roche LA, Steljes I, Janni W, Friedl TWP, De Wilde RL. The Association between Obesity and Premenopausal Breast Cancer According to Intrinsic Subtypes - a Systematic Review. Geburtshilfe Frauenheilkd. 2020 Jun;80(6):601-610. http://dx.doi.org/10.1055/a-1170-5004
[17] van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002 Jan 31;415(6871):530-6.http://dx.doi.org/10.1038/415530a
Figures
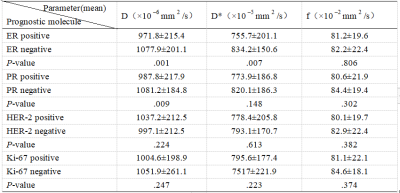
The IVIM parameters show that D value can distinguish ER and PR states, and D* can distinguish ER states (p <0.05), and the positive group is lower than the negative group (p <0.05), which is helpful to evaluate the prognosis molecular state of breast cancer and has certain guiding significance for the treatment of breast cancer.