4217
Diffusion Tensor Imaging of Swimmers’ Supraspinati: Fiber Distinctions Between Sprint and Distance Freestyle1Radiology, Stanford University, Stanford, CA, United States
Synopsis
Keywords: Muscle, Diffusion Tensor Imaging
Knowledge surrounding post-injury supraspinati in swimmers is vast, however a deep, preventative understanding at the cellular and fiber level is lacking. Through DTI, sixteen elite college swimmers were analyzed to evaluate mechanically stress induced differences between sprint and distance freestylers. Significant results (p-value<0.05) were found between groups for λ1, λ2, λ3, RD, MD, and volume. Findings indicated sprinters having a significantly larger MD and RD which was not compatible with the heightened levels microtrauma associated with aerobic distance training. DTI’s ability to inspect microstructural characteristics may pose it as an addition tool to conventional imaging techniques for athletes.
INTRODUCTION
In the sport of swimming and especially the freestyle stroke, supraspinatus tendinopathy or tendonitis of the supraspinatus is a prevalent injury due to the sport’s repetitive overhead motion and rigorous training regimen1. The supraspinatus is one of the four rotator cuff muscles in the shoulder, and it is responsible for initiating shoulder abduction or raising of the arm from the anatomical position2.More voluminous training is known to contribute to increase the prevalence of shoulder injuries, including supraspinatus tendinopathy. Traditionally, individual training volume is mainly based on race specialty such as sprint or distance. As distance swimmers commonly train further than sprinters to build their aerobic fitness3, they perform more strokes and cover more distance in training compared to sprinters, which could explain their higher rate of supraspinatus tendinopathy. A deeper understanding of differences between sprinter and long-distance swimmers could help optimize prevention and rehabilitation strategies, but this knowledge is currently lacking1.
Due to its excellent sensitivity to muscle cellular characteristics, Diffusion Tensor Imaging (DTI) may assess supraspinatus muscle composition at the cellular and fiber level to evaluate mechanical stress induced differences between these groups. In this work, we compare microstructural features of the supraspinatus in distance freestyle and sprint freestyle swimmers.
METHODS
Sixteen elite college swimmers (12 male and 4 female) were included in this study, after signing informed consent. Participants were assigned to either the sprint or distance group according to their three primary freestyle events. Swimmers whose primary events included the 500-yard freestyle or longer were assigned to the distance study group. Participant characteristics are noted in table 1.MRI evaluation was performed on a 3T scanner (GE HealthCare) with a 16-ch coil flex coil positioned inline from the sternum, around the shoulder, and to the scapula aiding data acquisition. The MRI protocol included a Dixon IDEAL sequence (for anatomical reference) and a DTI scan (DWI-EPI, TR/TE=3200/42.7ms, acquired resolution= 2.7x2.7x6 mm3, FOV=350x350x132 mm3, b-value = 400 s/mm2, 15 directions, NSA=3).
Total scanning time was approximately 10 minutes per subject. ITK-SNAP was utilized to segment the supraspinatus muscle from IDEAL water-only images4. Dipy was used for image preprocessing, tensor fitting, and extraction of DTI parameters (λ1, λ2, λ3, RD, MD, Fat Fraction, and FA)5,6. Correlations and differences between groups were assessed using multivariate linear regression. A p-value<0.05 was considered significant.
RESULTS
For the study group (sprint vs distance), BMI showed a significant positive correlation with RD (R2 = 0.470, p = 0.003), MD (R2 = 0.416, p = 0.007), λ2 (R2 = 0.487, p = 0.003), λ3 (R2 = 0.376, p = 0.012), and volume (R2 = 0.287, p = 0.033). Role (sprint or distance) was not a significant variable for any of the DTI parameters. However, when controlling for BMI, significantly higher values were found for RD (R2 = 0.657, p = 0.044), MD (R2 = 0.656, p = 0.021) and λ1 (R2 = 0.460, p = 0.032) in the sprinters compared to the distance swimmers.Supraspinatus stance volume did not differ significantly between sprinters and distance swimmers (p=0.914). Due to the small number of female athletes, we did not analyze the effect of sex on the quantitative metrics.
DISCUSSION
Data revealed a significantly larger RD and MD for sprinters compared to distance swimmers. This finding is not compatible with the presence of microtrauma in the distance swimmers, that in turn will increase their risk of overt injuries7, as in this case we would expect higher diffusion coefficients in the distance freestyle population.Sprinters are known to have a higher proportion of type II fiber compared to long distance swimmers8. Type II fibers are fast twitch muscle fibers that rely on anaerobic energy processes whereas type I fibers are slow twitch and rely on aerobic energy processes. Since type II fibers are larger in diameter than type I fibers, the increased diffusivity we observed in sprinters could reflect a different fiber type profile in these athletes. These findings will have to be confirmed with muscle biopsies.
While λ1 also showed significant differences between the two groups, when correcting for BMI, it is difficult to identify the cause of this finding. Due to the short diffusion times of our experiment, λ1 likely does not reflect differences in fiber length between the two groups.
CONCLUSION
The main finding of this study is the difference in MR, RD and λ1 in the supraspinatus between sprint and distance freestyle swimmers. Future research should validate our findings with gold-standard methods for fiber typing, and longitudinally follow the athletes in periods of low training and high training, as well water cross-training, to assess whether DTI parameters reflect acute or chronic effects of exercise.Despite these limitations, our study clearly reflects the ability of DTI to highlight microstructural characteristics of musculature in athletes that are not readily available using more conventional anatomical imaging.
Acknowledgements
No acknowledgement found.References
1. Sein ML, Walton J, Linklater J, Appleyard R, Kirkbride B, Kuah D, et al. Shoulder pain in elite swimmers: primarily due to swim-volume-induced supraspinatus tendinopathy. Br J Sports Med [Internet]. 2010 [cited 2022 Nov 1];44(2):105–13. Available from: https://doi.org/10.1136/bjsm.2008.047282
2. Pink M, Perry J, Browne A, Scovazzo ML, Kerrigan J. The normal shoulder during freestyle swimming. An electromyographic and cinematographic analysis of twelve muscles. Am J Sports Med [Internet]. 1991;19(6):569–76. Available from: https://doi.org/10.1177/036354659101900603
3. Pollock S, Gaoua N, Johnston MJ, Cooke K, Girard O, Mileva KN. Training regimes and recovery monitoring practices of elite British swimmers. J Sports Sci Med. 2019;18(3):577–85.
4. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;1;31(3):1116–28.
5. Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform. 2014 Feb 21;8.
6. Oudeman JM, Nederveen AJP, Strijkers GJP, Maas MMP, Luijten PRP, Froeling MP. Techniques and applications of skeletal muscle diffusion tensor imaging: A Review. 2015 [cited 2022 Nov 1]; Available from: https://doi.org/10.1002/jmri.25016
7. Hooijmans MT, Monte JRC, Froeling M, Berg‐Faay S, Aengevaeren VL, Hemke R, et al. Quantitative MRI Reveals Microstructural Changes in the Upper Leg Muscles After Running a Marathon. Journal of Magnetic Resonance Imaging. 2020 Aug 7;52(2):407–17.
8. Scheel M, von Roth P, Winkler T, Arampatzis A, Prokscha T, Hamm B, et al. Fiber type characterization in skeletal muscle by diffusion tensor imaging. NMR Biomed. 2013 Oct;26(10):1220–4.
Figures
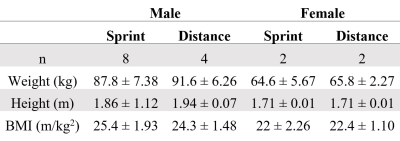
Table 1: Participant characteristics (mean ± SD).
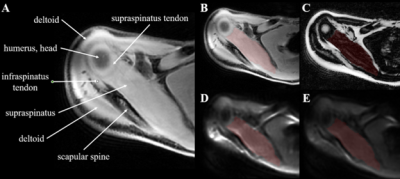
Figure 1: Scan A on the left depicts relevant anatomy and landmarks surrounding the supraspinatus. The right side of the figure displays 4 different scans of the same participant with the supraspinatus segmented in red: Water (B), Fat (C), DTI b=0 s/mm2 (D), and DTI b=400 s/mm2 (E).
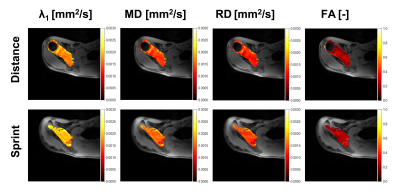

Figure 3: When controlling for BMI, sprinters had larger values for MD, RD, and λ1. Significant differences are indicated with an asterisk (*).