4211
1H-MRS human skeletal muscle quantification: a repeatability analysis
Alejandro Amador1,2 and Michael D. Noseworthy1,2,3,4
1School of Biomedical Engineering, McMaster University, Hamilton, ON, Canada, 2Imaging Research Centre, St. Joseph's Healthcare, Hamilton, ON, Canada, 3Electrical and Computer Engineering, McMaster University, Hamilton, ON, Canada, 4Department of Radiology, McMaster University, Hamilton, ON, Canada
1School of Biomedical Engineering, McMaster University, Hamilton, ON, Canada, 2Imaging Research Centre, St. Joseph's Healthcare, Hamilton, ON, Canada, 3Electrical and Computer Engineering, McMaster University, Hamilton, ON, Canada, 4Department of Radiology, McMaster University, Hamilton, ON, Canada
Synopsis
Keywords: Muscle, Spectroscopy
1H-MRS of skeletal muscle presents challenges due to the highly structured arrangement of muscle fibres and their orientation to B0, complicating metabolite visibility and quantification. This study aimed to assess the repeatability of 1H-MRS in the anterior tibialis muscle in healthy volunteers by acquiring multiple spectra during and within different scanning sessions. A robust method for positioning of the voxel-of-interest was used to ensure reproducible acquisition. Data analysis was done using LCModel. Statistical analysis showed no significant difference in metabolite ratios against water across sessions, suggesting good repeatability of 1H-MRS of skeletal muscle.Introduction
Proton magnetic resonance spectroscopy (1H-MRS) is a non-invasive technique used to assess body metabolism. Specifically, in skeletal muscle, it has been used to study the intramyocellular lipid (IMCL) content in healthy populations1, its relationship to insulin sensitivity2,3, as well as during exercise4,5. In addition to lipids, other metabolites such as creatine6, carnosine7 and acetyl-carnitine4 have been studied. Skeletal muscle 1H-MRS presents a series of challenges such as bulk magnetic susceptibility shifts and residual dipolar coupling effects due to the highly ordered structure, making visibility and quantification of metabolites orientation dependant (relative to B0), muscle dependant and voxel sensitive8. The aim of this study was to assess repeatability of 1H-MRS metabolite quantification of skeletal muscle in healthy subjects. To the authors knowledge, this has only been performed on lipids9 which have been reported to vary with diet and training status10.Methods
In a study approved by our IRB, scanning was performed with a GE Discovery MR750 3T MRI scanner. Five healthy male volunteers (age: 25±3yr, height: 178±2cm, weight: 77±8kg) were recruited. 1H-MRS (PRESS, VOI of 20x20x20mm3, TE/TR=30/1500ms, 256 averages) data were acquired from the anterior tibialis muscle using a 16-channel T/R extremity coil. VOI positioning was guided with axial proton density-weighted, fat-suppressed anatomical images (TE/TR/flip= 30/3000ms/111deg, 256x256 matrix, 4mm thickness, 15 slices).The protocol consisted of 30min resting in a supine position before scanning to allow for blood flow normalization11. Subsequently, six 1H-MRS spectra were acquired. The prescan values (shim, centre frequency, transmit/receive gains) were kept constant for all spectra based on values obtained from the first acquisition. Following these scans, two volunteers came back into the MRI 1-2 days later while the rest exited the magnet and were positioned back into the MRI 120 minutes later (1.5 hours free + 30min of supine resting) to simulate independent scans. Similar to the first scan, six more 1H-MRS were acquired during the second scan. To reproduce the same VOI for 1H-MRS in both sessions, a vitamin E pill was placed on the skin of the calf muscle (at the muscle belly) as a landmark (Fig.1).
To prevent any leg motion during the scan, sandbags and a Velcro leg strap were employed. An example of the VOI positioning is shown in Fig.1. Data were analyzed with LCModel12 (version 6.3): eddy current correction and water scaling were performed; water was used as internal reference8 and metabolite ratios were presented as a ratio relative to this peak. The muscle proton basis sets used were those included with LCModel, specifically for skeletal muscle. A repeated measures ANCOVA was performed to assess the differences in the mean metabolite ratio across scanning sessions, with the unsuppressed water peak full width at half maximum (FWHM), a metric indicative of shim quality, used as a covariate.
Results
A total of 90 1H-MRS spectra were collected from five volunteers across two sessions. An example 1H-MRS spectrum is shown in Fig.2. The creatine peak at 3.9ppm was excluded from the analysis due to residual dipolar coupling effects which split the resonance line. The average FWHM for the unsuppressed water peak was 13±0.5Hz across all spectra.A boxplot of the 12 metabolites included in the analysis is shown in Fig.3. Repeated measures ANCOVA was performed with metabolite, experimental session (2) and subject as factors, and the FWHM as confounding variable. Results showed no significant difference in the mean ratio of metabolites across experiment sessions (p=0.1848). On the other hand, the mean metabolite ratio between metabolites and subjects were statistically significant (p<0.001), as shown in Fig.4. Furthermore, multiple comparisons between scanning sessions for each metabolite showed a statistical difference only for the EMCL peak at 1.5ppm (Fig.5). Finally, the FWHM covariate was not significant (p=0.9502).
Discussion
The statistical difference between metabolites was expected since each metabolite shows a different concentration in skeletal muscle. Furthermore, previous studies have shown that metabolites change according to a person’s behaviour (e.g. diet and exercise), which explains the statistical difference between subjects. The FWHM (a measurement of the system shim and subsequent data quality) did not contribute any influence to the experimental design and thus can be disregarded. The mean metabolite ratios showed no statistical difference between scans, attributed to our robust and repeatable data acquisition strategy. However, even with our robust approach a statistical difference between scans for the EMCL – CH2 at 1.5ppm was noted, which has been reported to be highly dependent on VOI positioning.Conclusion
Our work showed high repeatability of skeletal muscle metabolites across scans of 1H-MRS in healthy subjects. It is thought that this was achieved by using a proper set-up, including the prevention of any leg motion and reproducible landmarks to place the VOI in the exact same location across sessions. Further studies should be conducted with a larger and sex-equilibrated population, with a longer time gap between sessions and controlling external factors such as diet and exercise to assess the overall repeatability and variability of 1H-MRS of skeletal muscle.Acknowledgements
The authors would like to thank the Image Research Centre (IRC) staff for their support with this study.References
1. Nakagawa Y, Hattori M. Intramyocellular lipids of muscle type in athletes of different sport disciplines. Open Access J Sports Med. 2017;8:161-166.2. Boesch C, Machann J, Vermathen P, Schick F. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed. 2006;19(7):968-988.
3. Klepochová R, Leutner M, Bastian M, et al. Muscle Specific Relation of Acetylcarnitine and Intramyocellular Lipids to Chronic Hyperglycemia: A Pilot 3T 1H MRS Study. Obes Silver Spring Md. 2020;28(8):1405-1411.
4. Ren J, Lakoski S, Haller RG, Sherry AD, Malloy CR. Dynamic monitoring of carnitine and acetylcarnitine in the trimethylamine signal after exercise in human skeletal muscle by 7T 1H-MRS. Magn Reson Med. 2013;69(1):7-17.
5. Vermathen P, Saillen P, Boss A, Zehnder M, Boesch C. Skeletal muscle 1H MRSI before and after prolonged exercise. I. muscle specific depletion of intramyocellular lipids. Magn Reson Med. 2012;68(5):1357-1367.
6. Kreis R, Jung B, Slotboom J, Felblinger J, Boesch C. Effect of Exercise on the Creatine Resonances in 1H MR Spectra of Human Skeletal Muscle. J Magn Reson. 1999;137(2):350-357.
7. Lievens E, Van Vossel K, Van de Casteele F, et al. CORP: quantification of human skeletal muscle carnosine concentration by proton magnetic resonance spectroscopy. J Appl Physiol. 2021;131(1):250-264.
8. Krššák M, Lindeboom L, Schrauwen-Hinderling V, et al. Proton magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed. 2021;34(5):e4266.
9. Torriani M, Thomas BJ, Halpern EF, Jensen ME, Rosenthal DI, Palmer WE. Intramyocellular Lipid Quantification: Repeatability with 1H MR Spectroscopy. Radiology. 2005;236(2):609-614.
10. Machann J, Stefan N, Schick F. 1H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol. 2008;67(2):275-284.
11. Elzibak AH, Noseworthy MD. Assessment of diffusion tensor imaging indices in calf muscles following postural change from standing to supine position. Magma. 2014;27(5):387-395.
12. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672-679.
Figures
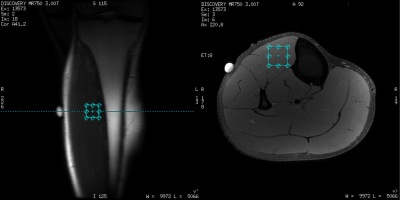
Figure 1. 1H-MRS VOI positioning was guided using an anatomical reference scan along with a vitamin E pill placed on the skin of the calf muscle at the muscle belly.
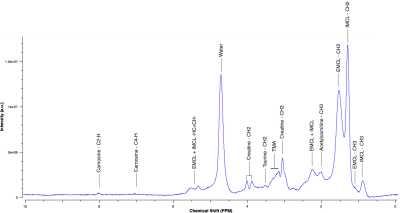
Figure 2. 1H-MRS muscle spectra of tibialis anterior (VOI of 20x20x20mm3, TE/TR=30/1500ms, 256 averages). Metabolites present in spectra were identified and labelled. TMA = trimethylamine complex. IMCL = intramyocellular lipids. EMCL = extramyocellular lipids.
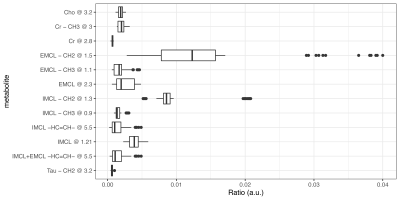
Figure 3. Exploratory boxplot of the metabolites included in the statistical analysis, such as creatine, choline, taurine, intramyocellular lipids (IMCL) and extramyocellular lipids (IMCL). EMCL – CH2 at 1.5ppm yielded the highest variability.
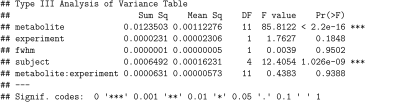
Figure 4. Repeated measurements ANCOVA results testing the mean metabolite ratio between metabolites, between experiments, and subject with the unsuppressed water peak full width at half maximum (FWHM), a metric indicative of shim quality, used as a covariate.
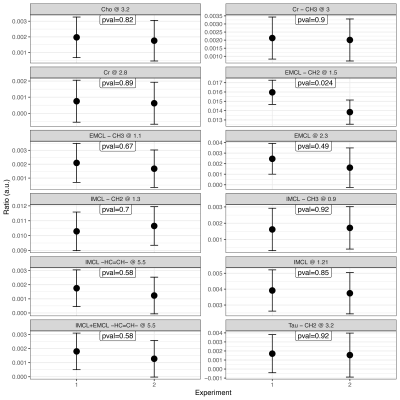
Figure 5. Multiple comparisons for each metabolite analyzed between scanning sessions. EMCL – CH2 at 1.5ppm was the only metabolite that showed a statistical difference (p=0.024) in the mean metabolite ratio between scanning sessions.
DOI: https://doi.org/10.58530/2023/4211