4201
Multi-Shot M1-Nulled Pancreatic Diffusion Weighted Imaging with Deep Learning-Based Denoising1Radiology, Stanford University, Palo Alto, CA, United States, 2GE HealthCare, Menlo Park, CA, United States, 3GE HealthCare, Houston, TX, United States
Synopsis
Keywords: Pancreas, Diffusion/other diffusion imaging techniques, Diffusion Weighted Imaging, Denoising, M1-nulled, ADC
Multi-shot DWI (msDWI) may be improved by M1 motion-compensation, but at a penalty of longer TE and lower SNR. DL-based denoising has recently emerged as an option for DWI, which could help offset this TE penalty. Here we assess the impact of a commercially available DL-based denoising tool in the setting of motion-compensated msDWI.INTRODUCTION
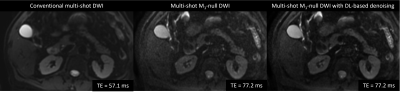
Diffusion Weighted Imaging (DWI) has the potential to add clinical value in the detection, diagnosis, and treatment of pancreatic cancer1-6. However, pancreatic DWI faces major challenge due to substantial variability and overlap in quantitative measurements such as apparent diffusion coefficient (ADC)7. Inaccurate ADC values may arise due to image distortion, insufficient signal-to-noise ratio (SNR), and/or image artifacts related to physiological motion (respiratory, peristaltic, and pulsatile)8. Multi-shot acquisition approaches have been shown to provide high-resolution DWI while limiting the impact of EPI-induced image distortions9. M1-nulled diffusion encoding has been shown to reduce bulk motion artifacts and improve the consistency of ADC quantification in single-shot DWI sequence10-12. However, this technique extends echo time (TE) substantially, thus reducing SNR and increases spatial blurring (Figure 1). Deep learning (DL) image reconstruction strategies have been shown to improve image sharpness and reduce noise in DWI13. Our objective was to evaluate the effect of DL-based denoising reconstruction on image quality, artifact, and ADC quantification of multi-shot pancreatic DWI with M1-nulled diffusion encoding.METHODS
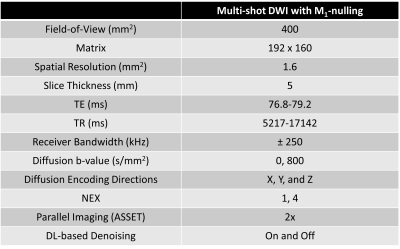
A total of 19 patients underwent abdominal MRI exams after IRB informed consent using 3T scanner (Premier, GE HealthCare, Waukesha, WI) with 30-channel phased array coils. Pancreatic DWI images were acquired using a custom respiratory triggered, multi-shot spin-echo EPI (SE-EPI) diffusion sequence with a real-time gradient optimization (GrOpt) framework14 that provided M1-nulled diffusion encoding waveforms (msDWI-M1). Imaging parameters are summarized in Table 1. The use of M1-nulled diffusion encoding waveforms extends the minimum achievable echo time (TE), which reduces SNR. Thus, images were reconstructed with and without a commercially available DL-based denoising reconstruction (AIR Recon DL, GE HealthCare, Waukesha, WI). To assess the effect of DL-based denoising to improve the image quality of the multi-shot M1-compensated diffusion encoding, we conducted an observer study performed by two radiologists, each with fellowship training in abdominal MRI and at least 7 years of radiology experience. Both radiologists were blinded to the information pertaining to patient’s clinical history, the image acquisition, as well as the image reconstruction. Analysis included blinded pair-wise comparisons of (i) pancreatic boundary delineation; (ii) perceived motion artifact in the pancreas; (iii) signal homogeneity in the pancreatic parenchyma; (iv) perceived noise in the pancreas; and (v) preference for reviewing the pancreas between msDWI-M1 with and without DL-based denoising (options: left images are better; images are equal; right images are better). All reviews were conducted on the high b-value (800s/mm2) diffusion images. Additionally, msDWI-M1 with DL-based denoising reconstruction were compared with a vendor-provided SE-EPI multi-shot DWI sequence (msDWI). To assess the impact of DL-based denoising on ADC quantification of the pancreas, ADC maps were computed from msDWI-M1 with and without DL-based denoising. A radiologist manually segmented the pancreatic head, body, and tail on each ADC map and computed the mean ADC values within each region. Repeated ANOVA was used to analyze ADC difference between the three pancreatic segments. If the ANOVA test showed statistically significant differences in ADC, subsequent post-hoc pairwise t-test with Bonferroni correction was used to test which pair of pancreatic segments had different ADC values.RESULTS
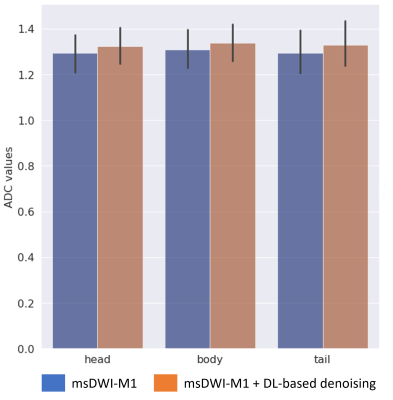
The readers observed less perceived noise in the pancreatic bed for msDWI-M1 with DL-based denoising than without DL-based denoising (reader 1: 58% and reader 2: 100% of cases). There was no substantial difference in perceived motion artifacts on msDWI-M1 whether DL-based denoising is applied or not. There was considerable disagreement between the two readers in term of signal homogeneity of the pancreatic parenchyma, delineation of pancreatic boundaries, and preference for reviewing the pancreas (Figure 3). Comparing msDWI with msDWI-M1 with DL-based denoising, both readers observed less noise in the pancreatic bed more frequently with the latter (36% and 47% of all cases). Furthermore, both readers observed increased homogeneity of the pancreas more frequently on msDWI-M1 with DL-based denoising (73% and 63% of all cases).In terms of ADC quantification, DL-based denoising did not significantly affect the measured ADC values in the pancreatic head, body, or tail (p>0.05). Furthermore, ADC values were not significantly different between the pancreatic head, body, and tail when compared with and without DL-based denoising (Figure 4).DISCUSSION
The use of msDWI-M1 with DL-based denoising effectively reduced the perceived noise in the pancreatic bed in most cases. However, there was considerable disagreement amongst the readers regarding pancreatic boundary delineation. This may partly be due to the fact radiologists are accustomed to reading images through certain level of noise in daily clinical practice. The use of msDWI-M1 increases the minimum achievable TE and reduces the SNR compared to non M1-nulled diffusion encoding. Our study suggests that by applying DL-based denoising technique, the increased TE and reduced SNR associated with msDWI-M1 may be mitigated, without impacting ADC quantification.CONCLUSION
DL-based denoising reconstruction reduced noise in the pancreatic bed while consistency of ADC quantification of the pancreas is preserved. This technique is potential useful in quantitative DWI using multi-shot M1-nulled DWI sequence.Acknowledgements
This project was supported, in part, by support from GE HealthCare.References
1. Akisik MF, Sandrasegaran K, Jennings SG, Aisen AM, Lin C, Sherman S, Rydberg MP. Assessment of chronic pancreatitis: utility of diffusion-weighted MR imaging with secretin enhancement. Radiology. 2009;250(1):103–109.
2. Lee SS, Byun JH, Park BJ, Park SH, Kim N, Park B, Kim JK, Lee MG. Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging. 2008;28(4):928–936.
3. Akisik MF, Aisen AM, Sandrasegaran K, Jennings SG, Lin C, Sherman S, Lin JA, Rydberg M. Diagnosis of chronic pancreatitis by using apparent diffusion coefficient measurements at 3.0-T MR following secretin stimulation. Radiology. 2009;252(2):418–425.
4. Zhu M, Zhang C, Yan J, Sun J, Zhao X, Zhang L, Yin L. Accuracy of quantitative diffusion-weighted imaging for differentiating benign and malignant pancreatic lesions: a systematic review and meta-analysis. European Radiology. 2021 Oct;31(10):7746-59.
5. Ren H, Mori N, Hamada S, Takasawa C, Mugikura S, Masamune A, Takase K. Effective apparent diffusion coefficient parameters for differentiation between mass-forming autoimmune pancreatitis and pancreatic ductal adenocarcinoma. Abdominal Radiology. 2021 Apr;46(4):1640-7.
6. Jeon SK, Jang JY, Kwon W, Kim H, Han Y, Kim D, Park D, Kim JH. Diffusion-weighted MR imaging in pancreatic ductal adenocarcinoma: prediction of next-generation sequencing-based tumor cellularity and prognosis after surgical resection. Abdominal Radiology. 2021 Oct;46(10):4787-99.
7. Geng R, Zhang Y, Starekova J, Rutkowski DR, Estkowski L, Roldán‐Alzate A, Hernando D. Characterization and correction of cardiovascular motion artifacts in diffusion‐weighted imaging of the pancreas. Magn Reson Med. 2021.
8. Hernando D, Zhang Y, Pirasteh A. Quantitative diffusion MRI of the abdomen and pelvis. Medical Physics. 2022 Apr;49(4):2774-93.
9. Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage. 2013;72:41-7. 10. Aliotta E, Wu HH, Ennis DB. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion–compensated diffusion weighted MRI. Magn Reson Med. 2017;77(2):717–729.
11. Zhang Y, Peña-Nogales Ó, Holmes JH, Hernando D. Motion-robust and blood suppressed M1-optimized diffusion MR imaging of the liver. Magn Reson Med. 2019;82(1):302–311
12. Sjölund J, Szczepankiewicz F, Nilsson M, Topgaard D, Westin CF, Knutsson H. Constrained optimization of gradient waveforms for generalized diffusion encoding. J Magn Reson. 2015;261:157–168 13. Lebel RM. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv preprint arXiv:2008.06559. 2020. 14. Loecher M, Middione MJ, Ennis DB. A gradient optimization toolbox for general purpose time‐optimal MRI gradient waveform design. Magn Reson Med 2020;84(6):3234-45.
Figures