4163
Does intravoxel incoherent motion MRI measure tumor perfusion? A comparison with DCE-MRI in patients with breast cancer.
Zyad M. Almutlaq1,2, Sarah E. Bacon3, Daniel J. Wilson3, Nisha Sharma4, and David L. Buckley1
1Biomedical Imaging, University of Leeds, Leeds, United Kingdom, 2Radiological Sciences Department, College of Applied Medical Sciences, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia, 3Department of Medical Physics & Engineering, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, 4Department of Radiology, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom
1Biomedical Imaging, University of Leeds, Leeds, United Kingdom, 2Radiological Sciences Department, College of Applied Medical Sciences, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia, 3Department of Medical Physics & Engineering, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, 4Department of Radiology, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, DSC & DCE Perfusion, IVIM
DCE-MRI can provide quantitative estimates of perfusion-related parameters in tumors, such as blood flow and blood volume fraction. It has also been proposed that IVIM MRI can be used to characterize perfusion in addition to microstructure. This proposal remains controversial and requires further investigation. In this study, we investigate the relationship between perfusion-related parameters measured by IVIM and DCE-MRI in a cohort of patients with breast cancer imaged before and after one and two cycles of neoadjuvant chemotherapy.Introduction
Dynamic contrast-enhanced (DCE)-MRI has a key role in the response monitoring of patients with breast cancer to treatment with neoadjuvant chemotherapy (NACT)1. DCE-MRI enables estimation of perfusion-related parameters of breast tumors, including blood flow (Fb) and blood volume fraction (vb)2. An alternative technique proposed to probe tumor perfusion is intravoxel incoherent motion (IVIM) diffusion-weighted imaging (DWI)3. It has been suggested that IVIM enables simultaneous assessment of tissue diffusion (Dt), pseudo-diffusion (Dp), perfusion fraction (f), and blood flow (f×Dp)4. However, previous studies that have explored the relationships between perfusion-related parameters measured by IVIM and DCE-MRI in a range of tumors have produced contradictory results5. The biophysical interpretation of the IVIM parameters f and f×Dp and their relationship to the DCE parameters Fb and vb remain controversial and require further investigation. This study aims to assess the correlations between these parameters in a cohort of patients with breast cancer imaged before and after one and two cycles of neoadjuvant chemotherapy.Methods
The study comprised 40 patients with primary breast cancer (mean age 46, range 25-69) due to undergo NACT. Patients were imaged on a 1.5 T MRI scanner (Aera; Siemens) before treatment, following one and three cycles of NACT. The scanning sequences included DWI with 6 b-values (0, 50, 100, 200, 400 and 800 s/mm2), a 3D IR-prepared spoiled gradient echo sequence to estimate T1 values (pre-and post-contrast) and interleaved high spatial and high temporal resolution (HSR and HTR , 2 s per volume) 3D spoiled gradient echo DCE sequences for tumor delineation and tracer kinetic analysis, respectively6,7. In addition to a 16-channel breast coil, a flexible array coil placed on the back was used to increase the signal from the descending aorta to enable accurate measurement of the arterial input function (AIF)8. Gd-DOTA was administered (0.1 mmol/kg) followed by saline (20 ml) at a rate of 3 ml/s.All MRI data were processed via in-house programs developed in MATLAB (Mathworks, USA). The DWI images (including ADC maps) were aligned to the corresponding HTR images and HTR and HSR subtraction images were generated. For each patient, a whole-volume region of interest (ROI) was generated using a 3D seeded region growing algorithm based on the threshold signal intensity (SI) of enhancing tumor in the HSR subtraction images. To reduce the possibility of tumor heterogeneity compromising the analysis, two sub-ROIs (5×5 pixels) of the whole-volume ROI were generated; the region with the lowest values on the ADC map (cold-spot ROI) and the region with the highest SI on the HTR subtraction (hot-spot ROI). All 3 ROIs were copied to the corresponding DWI, ADC, IR and HTR images.
For DCE, these three ROIs were used to generate SI-time curves from the HTR images and estimate T1 relaxation-times from the IR data. Further ROIs were drawn in the descending aorta for generating SI-time curves and estimating T1 before and after Gd-DOTA administration for AIF calculation8. SI-time data were converted to Gd-DOTA concentration-time using a bookend T1 correction8,9. A two-compartment exchange model was fitted to the DCE data and Fb, vb, capillary permeability-surface area product (PS) and extracellular-extravascular volume fraction (ve) were estimated10.
For DWI, the mean SI for each b‑value was extracted from the three tumor ROIs and fitted using a monoexponential and an IVIM model. The IVIM parameters were estimated using an over-segmented approach (estimating Dt and f from the high b-value data, then fixing Dt and f and estimating Dp)11. A Spearman rank test was used to assess the correlation between the diffusion and DCE parameters.
Results
When data from all 3 visits were combined there were moderate positive correlations between the diffusion parameters ADC & Dt and the DCE parameter ve estimated in whole-tumor ROIs (r = 0.493 and 0.505 for ADC & Dt, respectively; n = 74, P<0.001) (Figure 1). However, there were no significant correlations between the IVIM parameters f & f×Dp and the DCE parameters Fb & vb. Significant correlations between these parameters were only seen in selected subsets of the combined data (e.g., a moderate correlation between f and Fb following cycle 1 in hot-spot ROIs; r = 0.559, n = 32, P = 0.001, Figure 2). This correlation was not maintained across other tumor ROIs or treatment time-pointsDiscussion and conclusion
Our findings of the moderate correlations between the diffusion parameters (ADC, Dt) and ve suggest that water diffusion increases as the interstitial fluid volume increases, an expected characteristic of the diffusion signal but one that has been challenged12. However, our data did not identify any consistent correlations between perfusion parameters estimated by IVIM and DCE. We recognize that the precision with which parameters such as vb and f×Dp are estimated is poor but estimates of f and Fb are more precise and our data support the suggestion that a single diffusion coefficient may not reflect the complex diffusion properties of the vascular signal13.Acknowledgements
The study was funded by Breast Cancer Now (award 2014MayPR241).References
1. Shenoy H, Peter M, Masannat Y, Dall B, Dodwell D, Horgan KJSO (2009) Practical advice on clinical decision making during neoadjuvant chemotherapy for primary breast cancer. Surgical Oncology 1:65-712. Georgiou L, Sharma N, Broadbent DA et al (2018) Estimating breast tumor blood flow during neoadjuvant chemotherapy using interleaved high temporal and high spatial resolution MRI. Magnetic Resonance in Medicine 79:317-326
3. Iima M, Honda M, Sigmund EE, Ohno Kishimoto A, Kataoka M, Togashi K (2020) Diffusion MRI of the breast: Current status and future directions. Journal of Magnetic Resonance Imaging 52:70-90
4. Le Bihan D, Turner R (1992) The capillary network: a link between IVIM and classical perfusion. Magnetic Resonance in Medicine 27:171-178
5. Federau C (2017) Intravoxel incoherent motion MRI as a means to measure in vivo perfusion: A review of the evidence. NMR in Biomedicine 30:e3780
6. Stevens W, Farrow IM, Georgiou L et al (2021) Breast tumour volume and blood flow measured by MRI after one cycle of epirubicin and cyclophosphamide-based neoadjuvant chemotherapy as predictors of pathological response. The British journal of radiology 94:20201396
7. Almutlaq ZM, Wilson DJ, Bacon SE et al (2022) Evaluation of monoexponential, stretched‐exponential and intravoxel incoherent motion MRI diffusion models in early response monitoring to neoadjuvant chemotherapy in patients with breast cancer—A preliminary study. Journal of Magnetic Resonance Imaging 56:1079-1088
8. Georgiou L, Wilson DJ, Sharma N, Perren TJ, Buckley DL (2019) A functional form for a representative individual arterial input function measured from a population using high temporal resolution DCE MRI. Magnetic Resonance in Medicine 81:1955-1963
9. Cron GO, Santyr G, Kelcz F (1999) Accurate and rapid quantitative dynamic contrast‐enhanced breast MR imaging using spoiled gradient‐recalled echoes and bookend T1 measurements. Magnetic Resonance in Medicine 42:746-753
10. Sourbron S, Buckley DL (2011) Tracer kinetic modelling in MRI: estimating perfusion and capillary permeability. Physics in Medicine and Biology 57:R1
11. Suo S, Lin N, Wang H et al (2015) Intravoxel incoherent motion diffusion‐weighted MR imaging of breast cancer at 3.0 tesla: comparison of different curve‐fitting methods. Journal of Magnetic Resonance Imaging 42:362-370
12. Arlinghaus LR, Li X, Rahman AR et al (2011) On the relationship between the apparent diffusion coefficient and extravascular extracellular volume fraction in human breast cancer. Magnetic Resonance Imaging 29:630-638
13. Zhang X, Ingo C, Teeuwisse WM, Chen Z, van Osch MJ (2018) Comparison of perfusion signal acquired by arterial spin labeling–prepared intravoxel incoherent motion (IVIM) MRI and conventional IVIM MRI to unravel the origin of the IVIM signal. Magnetic Resonance in Medicine 79:723-729
Figures
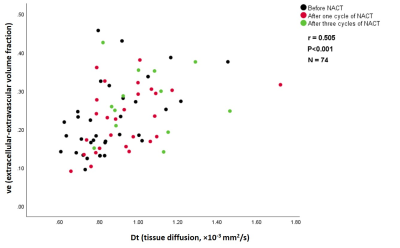
Figure 1. A scatter plot
shows a moderate positive correlation between the IVIM parameter (Dt) and the
DCE parameter (ve) estimated in whole-tumor ROIs of combined data from all three
visits (before treatment, following one and three cycles of NACT).
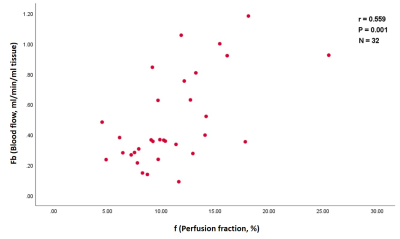
Figure 2. A scatter plot
shows a moderate positive correlation between the IVIM parameter (f) and DCE
parameter (Fb) estimated in hot-spot ROIs following one cycle of NACT.
DOI: https://doi.org/10.58530/2023/4163