4161
Age-related time-dependent diffusivity changes within healthy human prostate1Department of Biomedical Engineering, University of Alberta, Edmonton, AB, Canada, 2Department of Urology, University of Alberta, Edmonton, AB, Canada, 3Department of Radiology, University of Alberta, Edmonton, AB, Canada, 4Siemens healthineers, Erlangen, Germany
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Prostate
Diffusion MRI is used daily in prostate cancer diagnosis. Modification of the diffusion time can probe alterations in prostate microstructure dimensions that may occur with aging or cancer. The mean diffusivity (MD) difference between ‘long’ diffusion time stimulated echo acquisition mode (STEAM) and ‘short’ diffusion time oscillating gradient spin echo (OGSE), and between STEAM and ‘medium’ diffusion time pulsed gradient spin echo (PGSE) showed significant positive linear correlation versus age in peripheral zone of healthy adult prostate (n=15); however, this correlation was not identified in the central gland. Diffusion time sensitivity could reveal age-related prostate tissue microstructural remodeling.Introduction
Diffusion MRI has shown age-related increases of mean diffusivity (MD) in healthy adult prostate with a greater slope in the peripheral zone (PZ) than the central gland (CG) that potentially relates to glandular density changes with age1. Age-related glandular density change may be related to the incidence of prostate cancer2. Alterations of prostate micro-structure (‘healthy’ or tumor) dimensions can be interrogated by comparing diffusion parameters acquired with different diffusion times – short (e.g., 6 ms) with oscillating gradient spin echo (OGSE), medium (e.g., 40 ms) with typically used pulsed gradient spin echo (PGSE) and long (e.g., 100 ms) with stimulated echo acquisition mode (STEAM). STEAM diffusion MRI was used to show marked MD reductions with increased diffusion time from 21 to 350 ms in prostate tumors with comparably less diffusion time effects in benign zones3. OGSE and PGSE diffusion MRI were used to vary diffusion times from 7.5 to 30 ms that enabled microstructural modeling in prostate cancer and showed greater diffusion time dependence in higher Gleason Grade tumors4. The purpose here was to compare diffusion time (6 to 100 ms) effects in the healthy prostate with aging by acquiring OGSE, conventional PGSE and STEAM, which will also serve as a benchmark for future diffusion time comparisons to prostate cancer (factoring in age).Methods
Healthy male volunteers (n=15, 27-63 years) were scanned on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). OGSE, PGSE, and STEAM diffusion MRI were acquired with a single shot EPI research application with the following common parameters: 10 axial slices with the middle slice positioned in the center of the prostate, 3 mm thick (no gap), 2x2 mm2 in-plane resolution, GRAPPA R=2, TR 3000 ms, 15 b0, 6 directions at b=500 s/mm2 each with 15 averages, phase encoding A>>P, and 5:34 min acquisition time per diffusion scan. The TE was 87 ms for OGSE and PGSE and 60 ms for STEAM. Diffusion times were ~ 6 ms for OGSE (trapezoid cosine 40 Hz), 40 ms for PGSE, and 100 ms for STEAM. All three scans gave the same MD values for phantoms of n-nonane (MD=1.63+/-0.015x10-3 mm2/s) and n-decane (MD=1.27+/-0.022x10-3 mm2/s), chosen since their MDs are similar to PZ and CG, respectively. An additional high-resolution 0.75x0.75x1.8 mm3 T2-weighted image (40 axial slices, 7 min) was acquired for anatomical comparison. Image preprocessing was done using DIPY including MPPCA denoising, symmetric diffeomorphic registration. Manual regions of interest were identified on mean b500 image over multiple slices within peripheral zone (PZ) and central gland (CG) for comparison of OGSE, PGSE, and STEAM MD values. Pairwise group differences of MD were assessed across all three diffusion time scans using a paired T-Test. Linear regression versus age was assessed for MD values for each scan and OGSE-STEAM, PGSE-STEAM, and OGSE-PGSE MD differences.Results
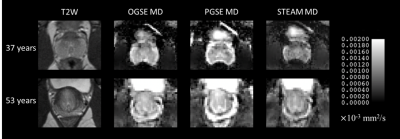
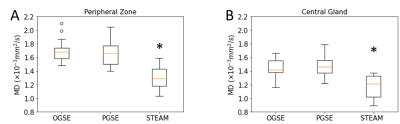
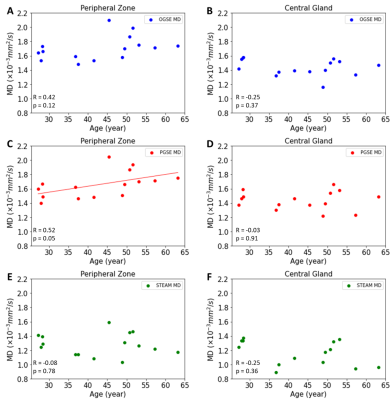
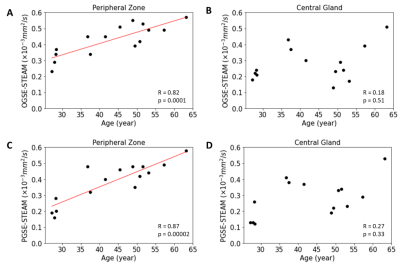
The MD maps showed that long diffusion time (100 ms) STEAM MD is significantly lower than short diffusion time (6 ms) OGSE MD and medium diffusion time (40 ms) PGSE MD (Figure 1). There were no MD differences between OGSE and PGSE in either PZ (p=0.49) or CG (p=0.99), but MD was significantly lower for STEAM by 24% in the PZ (p=0.0000001 vs OGSE; p=0.000001 vs PGSE) and 14% in the CG regions (p=0.0005 vs OGSE, p=0.0006 vs PGSE), respectively (Figure 2). The MD of PGSE in the peripheral zone was positively correlated to age, whereas no such correlations were observed in the central gland. No significant correlation was identified for OGSE MD or STEAM MD versus age in either PZ or CG (Figure 3). OGSE-STEAM MD and PGSE-STEAM MD difference versus age showed a significant strong linear positive correlation in PZ; however, no significant correlations were identified in the CG (Figure 4). Notably, OGSE-PGSE MD difference versus age showed no significant correlation in either PZ or CG (PZ p=0.32; CG p=0.13; data not shown).Discussion
Diffusion time effects on MD were confirmed in healthy prostate, but this was only for the long diffusion time STEAM MD which was 24% lower relative to the short OGSE and medium PGSE MD in the PZ and 14% lower in the CG; in contrast the OGSE and PGSE MD values did not differ in either region. A previous study using only STEAM is in partial agreement in that they showed long diffusion time effects in benign PZ, albeit a much smaller effect than we see in healthy controls, but not in benign transition zone (part of CG) in prostate cancer patients3. Further, OGSE at 17 and 33 Hz appear to give similar diffusion coefficients as PGSE (0 Hz) in benign tissue of prostate cancer patients4 in agreement with our results. The strong positive linear correlations between both OGSE-STEAM and PGSE-STEAM MD differences versus age (27-63 years) in PZ in healthy prostate is a new observation which suggests a fundamental diffusion time sensitivity to age related micro-structural changes presumed to be from decreases in glandular and cellular density. As 80% of prostate cancer originates from the glandular epithelium within the peripheral zone, this observed phenomenon may explain the inverse relationship between benign prostatic hyperplasia and incidence of prostate cancer2.Acknowledgements
References
1. Tamada T, Sone T, Toshimitsu S, Imai S, Jo Y, Yoshida K, Yamamoto A, Yamashita T, Egashira N, Nagai K, Fukunaga M. Age-related and zonal anatomical changes of apparent diffusion coefficient values in normal human prostatic tissues. J Magn Reson Imaging. 2008 Mar;27(3):552-6. doi: 10.1002/jmri.21117. PMID: 18219616.
2. Weaver PE, Smith LA, Sharma P, Keesari R, Al Mekdash H, de Riese WT. Quantitative measurements of prostate capsule and gland density and their correlation to prostate size: possible clinical implications in prostate cancer. Int Urol Nephrol. 2020 Oct;52(10):1829-1837. doi: 10.1007/s11255-020-02527-6. Epub 2020 Jun 6. PMID: 32506207.
3. Lemberskiy G, Rosenkrantz AB, Veraart J, Taneja SS, Novikov DS, Fieremans E. Time-Dependent Diffusion in Prostate Cancer. Invest Radiol. 2017 Jul;52(7):405-411. doi: 10.1097/RLI.0000000000000356. PMID: 28187006.
4. Wu D, Jiang K, Li H, Zhang Z, Ba R, Zhang Y, Hsu YC, Sun Y, Zhang YD. Time-Dependent Diffusion MRI for Quantitative Microstructural Mapping of Prostate Cancer. Radiology. 2022 Jun;303(3):578-587. doi: 10.1148/radiol.211180. Epub 2022 Mar 8. PMID: 35258368.
Figures