4157
Multidimensional diffusion MRI for monitoring radiotherapy response in human prostate cancer xenografts in mice: A longitudinal pilot study
Filip Szczepankiewicz1, Marcella Safi2, Crister Ceberg1, Michael Gottschalk3, Evangelia Sereti4, Anders Bjartell4, Oskar Vilhelmsson Timmermand5, Linda Knutsson1,6, Sven-Erik Strand1,7, and Joanna Strand2,7
1Medical Radiation Physics, Lund University, Lund, Sweden, 2Hematology, Oncology, Radiation Physics, Lund University, Lund, Sweden, 3Lund University Bioimaging Centre, Lund University, Lund, Sweden, 4Translational Medicine, Lund University, Lund, Sweden, 5King's College London, London, United Kingdom, 6F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 7Dept of Oncology, Lund University, Lund, Sweden
1Medical Radiation Physics, Lund University, Lund, Sweden, 2Hematology, Oncology, Radiation Physics, Lund University, Lund, Sweden, 3Lund University Bioimaging Centre, Lund University, Lund, Sweden, 4Translational Medicine, Lund University, Lund, Sweden, 5King's College London, London, United Kingdom, 6F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 7Dept of Oncology, Lund University, Lund, Sweden
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Microstructure, Prostate Cancer, Multidimensional MRI, Radiotherapy
We use multidimensional diffusion MRI to monitor longitudinal effect of external radiotherapy in human prostate cancers in mice. The measured dimensions are “diffusion time” and “shape of the b-tensor,” enabling a probe of tissue heterogeneity, microscopic anisotropy and restriction sizes. We show that the diffusivity is highly time dependent in all tumors, and that it is significantly altered by radiotherapy, already within 1-15 days of treatment. The largest effect is seen for the diffusivity and its time dependence, but the isotropic diffusional variance is also impacted. Throughout, histology is used qualitatively to provide plausible interpretations to the observations.Introduction
Multidimensional MRI leverages joint measurements across multiple dimensions and is especially promising in heterogeneous tissue such as prostate cancer. However, the most relevant dimensions are not yet established. A promising dimension is that of “b-tensor shape,” however, the associated gradient waveforms also change the diffusion time which may confound the measurement[1,2]. Instead of avoiding this contrast, we aim to exploit it[3-5] and evaluate if can contribute potential biomarkers for prostate cancer monitoring.$$$~~~~$$$We propose a measurement and analysis that enables an efficient and joint modulation of the b-tensor shape and diffusion time[3-5], and we use it to monitor the effect of external radiotherapy of human prostate cancer in mice. We explore biomarker candidates for longitudinal monitoring of prostate cancer during/after radiotherapy and compare them to the underlying histology.
Methods
Mouse modelFour nude mice (BALB/c, Foxnnu/nu) were subcutaneously inoculated with human prostate cancer cells (LNCaP) in the right flank (5-7·106 cells). The study was in accordance with national and local ethics regulations. The animals were monitored for tumor volume (caliper and ultrasound), body weight and signs of illness. After the final MRI, the mice were sacrificed, and tumors were dissected.
Radiotherapy
External beam irradiation was performed under isoflurane anesthesia with a small animal radiotherapy system (Xstrahl XenX with average photon energy of 78$$$~$$$keV). The absorbed dose was 10$$$~$$$Gy to the whole tumor delivered in a single square field tangential to the body.
Longitudinal MRI
MRI was performed at 9.4 T (Bruker BioSpec Avance III) with a 10 or 20$$$~$$$mm surface coil. Each mouse was scanned under isoflurane anesthesia at four timepoints: one day before, 2-3 days after, 9-10 days after, and 14-15 days after radiotherapy.
Morphological imaging was performed with a RARE sequence with TE=30$$$~$$$ms, TR=2.5$$$~$$$s, voxel size=0.125×0.125×1.00$$$~$$$mm3, for a total of 5 min.
dMRI was performed with a sequence that enables user defined gradient waveforms (see Acknowledgements). Parameters were TE=37$$$~$$$ms, TR=2.2$$$~$$$s, and voxel size=0.250×0.250×1.00$$$~$$$mm3. We used four gradient waveforms to yield spherical, planar and linear b-tensors (see below), executed at b=[0.1$$$~$$$0.7$$$~$$$1.4$$$~$$$2.0] ms/µm2 with 30 rotations each, with a total scan time of 18 min.
Gradient waveform design
A single waveform for spherical b-tensor encoding was optimized for minimal encoding times at 500 mT/m[6]. To force a convenient symmetry in the gradient waveforms, we imposed a novel optimization constraint such that one axis was time-symmetric (gx(t)=gx(-t)), whereas gy(t) and gz(t) were time-reversed (gy(t)=gz(-t)). This yields long diffusion times on the symmetry axis (x) and short diffusion times on the orthogonal axes. Waveforms for linear and planar b-tensor encoding were subsets of the first, as detailed in Fig.1.
dMRI parameter estimation
We fitted a signal representation inspired by[3,4,7] to capture diffusion time effects as a function of the variance of the dephasing vector power spectrum (ω, Fig.1) and b-tensor shape (bΔ)[8]$$S~=~S_0~\mathrm{exp}[-b(D+D_ω\cdotω)+b^2(V_I+V_{Iω}\cdotω^2+b_Δ^2(V_A+V_{Aω}\cdotω^2))/2]$$where D+Dω·ω is the observed diffusivity at a given ω, and similarly, VI and VA are the isotropic and anisotropic diffusion variances[9,10] with corresponding diffusion time dependent contributions. For simplicity, the effect of changing ω is presented as the absolute change (prefix Δ) observed across the range of employed ω. For example, ΔD is the diffusivity change as ω goes from 1.2·104 to 10.0·104 s-2. dMRI parameters were evaluated as averages over tumor ROIs. Additionally, the tumor in mouse 3 contained two distinct microstructure subtypes which were analyzed independently.
Histology
Excised tumors were frozen and sent for immunohistochemistry and microscopy. Samples were sectioned at 4 µm and stained with H&E and prostate-specific membrane antigen (PSMA). Slices were digitally scanned and investigated qualitatively; slices from histology and MRI were not matched.
Results and discussion
The radiotherapy had a clear effect on tumor growth and morphology (Fig.2), however the effects manifested differently both within and between subjects. For example, the tumor in mouse 2 remained homogeneous, whereas the tumor in mouse 4 became highly necrotic and liquid.$$$~~~~$$$Fig.3 and 4 show parameter maps and parameters-vs-time, respectively. Significant trends were seen for D, ΔD, VI and ΔVI, whereas the diffusion anisotropy remained low and relatively unchanged. The elevated diffusivity is expected from previous investigations radiotherapy effects[11,12]. Throughout subjects, ΔD is only elevated in tumor tissue, and virtually zero elsewhere. Strikingly, the effect of modulated diffusion time on diffusivity is approximately 50-100%, indicating that structural sizes in tumors are commensurate with the distance probed by the diffusing water.
$$$~~~~$$$The two tumor regions in mouse 3 (Fig.5) started out with similar diffusion features but diverged after treatment; the histologically dense tissue trended toward lower D, higher ΔD, and lower VI. Although the mechanism is yet to be explored, this difference may facilitate biomarkers that distinguish regional response to treatment.
$$$~~~~$$$This study has several limitations: small group, low SNR at high-b, potential exchange/micro-kurtosis effects[4,13], and histology and MRI in different spaces). Nevertheless, we emphasize the value of longitudinal investigations with carefully administered treatment as a means to explore biomarker candidates.
Conclusions
- We proposed a joint measurement/analysis of diffusional variance, microscopic anisotropy, and diffusion time dependence.
- We observed a marked diffusion time dependence in cancer tissue, mainly in the diffusivity and diffusional variance.
- Radiotherapy induced a significant change in dMRI parameters within 1-15 days; the effect was heterogeneous across/within tumors.
Acknowledgements
We thank Mathew Budde for generously providing the Bruker pulse sequence which can be found at https://osf.io/t9vqn/. We thank Bo Holmqvist at ImaGene-iT AB (Medicon Village, Lund, Sweden) for performing the histology analysis. This research was funded by the Swedish Cancer Society (22 0592 JIA), Swedish Research Council (2021-04844), the Swedish Prostate Cancer Federation, Mrs. Berta Kamprad's Foundation (FBKS-2021-24-(344) and FBKS-2022-41-(425)) and the ALF Foundation of the Medical Faculty of Lund University.References
- Szczepankiewicz, F., C.F. Westin, and M. Nilsson, Gradient waveform design for tensor-valued encoding in diffusion MRI. J Neurosci Methods, 2021: p. 109007.
- Jespersen, S.N., et al., Effects of nongaussian diffusion on "isotropic diffusion" measurements: An ex-vivo microimaging and simulation study. J Magn Reson, 2019. 300: p. 84-94.
- Narvaez, O., et al., Massively Multidimensional Diffusion-Relaxation Correlation MRI. Frontiers in Physics, 2022. 9.
- Chakwizira, A., et al., Diffusion MRI with pulsed and free gradient waveforms: Effects of restricted diffusion and exchange. NMR Biomed, 2022: p. e4827.
- Nilsson, M., et al., Resolution limit of cylinder diameter estimation by diffusion MRI: The impact of gradient waveform and orientation dispersion. NMR Biomed, 2017. 30(7).
- Szczepankiewicz, F., C.F. Westin, and M. Nilsson, Maxwell-compensated design of asymmetric gradient waveforms for tensor-valued diffusion encoding. Magn Reson Med, 2019.
- Nilsson, M., et al. A unified framework for analysis of time-dependent diffusion: numerical validation of a restriction-exchange correlation experiment. in Proc. Intl. Soc. Mag. Reson. Med. 28. 2020. Virtual.
- Westin, C.F., et al., Q-space trajectory imaging for multidimensional diffusion MRI of the human brain. Neuroimage, 2016. 135: p. 345-62.
- Lasič, S., et al., Microanisotropy imaging: quantification of microscopic diffusion anisotropy and orientational order parameter by diffusion MRI with magic-angle spinning of the q-vector. Frontiers in Physics, 2014. 2: p. 11.
- Szczepankiewicz, F., et al., The link between diffusion MRI and tumor heterogeneity: Mapping cell eccentricity and density by diffusional variance decomposition (DIVIDE). Neuroimage, 2016. 142: p. 522-532.
- Takayama, Y., et al., ADC value and diffusion tensor imaging of prostate cancer: changes in carbon-ion radiotherapy. J Magn Reson Imaging, 2008. 27(6): p. 1331-5.
- Park, S.Y., et al., Early changes in apparent diffusion coefficient from diffusion-weighted MR imaging during radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys, 2012. 83(2): p. 749-55.
- Henriques, R.N., S.N. Jespersen, and N. Shemesh, Correlation tensor magnetic resonance imaging. Neuroimage, 2020. 211: p. 116605.
Figures
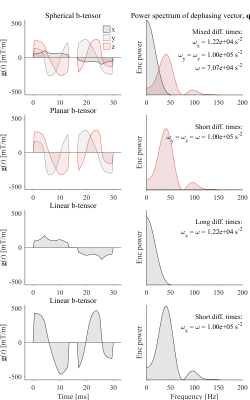
Fig.1 - Gradient waveforms for spherical b-tensor encoding (top row) were numerically optimized[6] with additional constraints on symmetry. Remaining waveforms were subsets: planar is the y-z-axes, linear with long and short diffusion times are the x and y axes, respectively. The right collumn shows the power spectra of the dephasing vector q(t); high power at high frequencies results in high ω and short diffusion times, and vice versa.
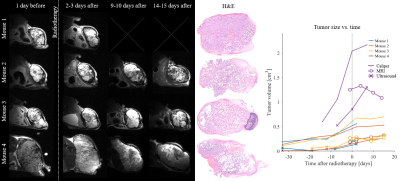
Fig.2 - Morphological images at each time point before and after radiotherapy (10 Gy at time zero) and the corresponding tumor volume estimates based on caliper measurements, ultrasound (US) and MRI. The radiotherapy effects are clear but heterogeneous. The growth of tumors was siezed, and gross morphological changes can be seen by MRI and histology (only H&E stain is shown). Note that mouse 1 was sacrificed the day after the second MRI due to poor health.
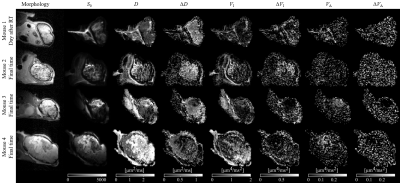
Fig.3 - Diffusion parameter maps at the last time point available. Tumors exhibit differnt parameters, however, elevated time dependency is specific to tumor tissue (potentially also the skin proximal to the tumor). The tumor in mouse 2 remains relatively homogeneous, mouse 3 exhibits two distinct tumor regions (Fig.5), and mouse 4 has a tumor that becomes liquid. Diffusion anisotropy (VA and ΔVA) was low, although mouse 3 and 4 exhibit non-zero VA in parts of the tumor. Variance parameters were markedly noisy, and may be affected by noise floor effects due to poor SNR at high b-values.
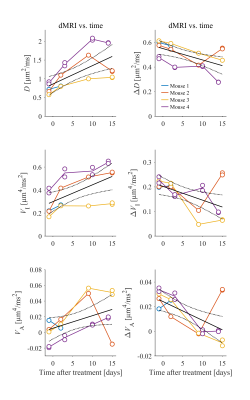
Fig.4 - Diffusion parameters averaged across the entire tumor ROIs vs. time after treatment. Note that the first MRI is performed one day before treatment, and the treatment is at time zero. A linear regression shows significant trends for diffusivity and isotropic variance (top four plots). We observe that the measurements are highly repeatable; doublets of data markers (e.g. all time points for mouse 4) are close, and sometimes overlapping.

Fig.5 - A closer inspection of the tumor in mouse 3 shows that it had two distinct regions. The dMRI parameters in the two regions are similar prior to treatment, but diverge after radiotherapy. The trend is that the dense tissue (red lines) has lower D, higher ΔD, and lower VI; in histology it is observed to contain a dense cell matrix with smaller cells with less expression of the prostate specific membrane antigen (PSMA). The zoomed histology frames are at the same magnification.
DOI: https://doi.org/10.58530/2023/4157