4152
Aquaporin4 inhibitor alters the apparent diffusion coefficients in mouse brain1National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan, 2Faculty of Engineering, University of Tsukuba, Tsukuba, Japan, 3Sorbonne University, Paris, France, 4Central Institute for Experimental Animals, Kawasaki, Japan
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Diffusion/other diffusion imaging techniques
The glymphatic system seems to be involved to protect the brain pathologies like Alzheimer’s disease, by the clearance of brain waste1. A previous study showed the apparent diffusion coefficients (ADC) in several brain regions were increased by aquaporin 4 (AQP4) inhibitor2. However, the time course of ADC change has not been investigated. In this study, we showed the time course of ADC change in multiple mouse brain regions following injection of the TGN020 and the effect of TGN020 on volume-dependent intrinsic optical signals in brain slices.Introduction
The glymphatic system in the brain is regulated through aquaporin 4 (AQP4) in the astrocytes. Especially, AQP4 is essential in clearing brain wastes, such as Amyloid-beta, which are related to Alzheimer’s disease1. A previous study showed the apparent diffusion coefficients (ADC) in several brain regions were increased by AQP4 inhibitor2. However, the time course of ADC has not been investigated. In this study, using diffusion-weighted imaging (DWI), we investigated the time course of ADC change following injection of the TGN020 sodium salt or saline. The ex vivo brain slice was also used to investigate the volume change of the astrocytes by TGN020.Ten C57BL6 mice were used for the experiment. MRI experiment was performed with medetomidine anesthesia (0.6 mg/kg/h, i.v.). The mice were injected with TGN020 and saline at 4 weeks intervals. For the crossover test, the order of injection of TGN020 or saline was randomly determined. The respiration was monitored, and the body temperature was maintained at 36 °C by circulating hot water during the measurement.
MRI experiment
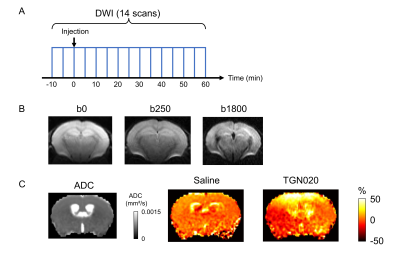
The MRI experiments were conducted on a Bruker 7T scanner with a cryo-cooled mouse brain coil. After 10 min from the start of the scanning, TGN020 (250 mg/kg body weight) or Saline was injected. Then, scanning was continued for 60 min (Fig. 1A). The body temperature was maintained at 36 °C via the circulating hot water. We used a standard diffusion-weighted spin echo EPI sequence, with the following parameters: spatial resolution = 175 x 175 x 500 μm3, 3 b-values (b = 0, 250 and 1800 s/mm2), 6 directions, 1 segment, TE = 37.1 ms / TR = 5769 ms, bandwidth = 300 kHz, δ = 3 ms, Δ = 24 ms, total scan time = 5 min (Fig. 1B). The DWI scan was continued for 70 min (total 14 scans).
Data analysis
The ADC at each time point was calculated from DWI data (Fig. 1C). The percent change of ADC was then calculated following equation, ΔADCi = (ADCi / ADC2 - 1) x 100 (%). The ADCi is the ADC at the i-th timepoint. We define the ADC at 2nd time-point as the baseline because the injection was performed after 2nd DWI scan (Fig. 1A). The averaged ADC changes within the regions of interest (ROIs) were calculated using a homemade program.
Slice preparation and intrinsic optical imaging (IOS)
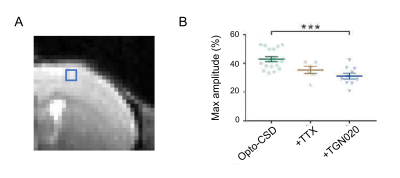
The 18 to 20-day-old heterozygous Emx1-crecre/WT::Ai32ChR2/WT mice, in which channelrhodopsin 2 (ChR2) is expressed in pyramidal cells were used. A 10s continuous photostimulation at 470 nm of the ChR2H134R was carried out on the ex vivo slice (300 μm thick) using a collimated LED system, and the change of light transmittance by extracellular space change was investigated. To assess the contribution of AQP4 in astrocyte volume, 20 μM TGN020 was applied. Tetrodotoxin (TTX, 1 µM) was used to block the action potentials of the neurons.
IOS analysis
The transmittance variations (ΔT) or IOS were determined according to: ΔT = (Ti-T0) / T0 where Ti transmittance at a time t and T0 is the average transmittance during the first minute of the baseline period. The parameters analyzed were determined from the transmittance variation curves [(ΔT/T0) = f(t)]. Optogenetically-induced cortical spreading depressions3 (Opto-CSD) were considered successful if the maximum rise rate of IOS exceeded 2%/second4.
Results and Discussion
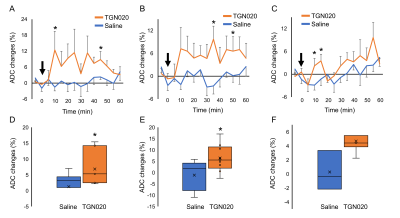
Figure 1C shows the ADC changes in a representative mouse 55-60 min after injection of saline or TGN020. The ADC in the cortex, the ventricle, and the striatum was increased over time by TGN020 injection. ADC in the cortex and the striatum started to increase 10 min following the injection of TGN020 and this increase was prolonged until 60 min, while no effect was observed with the injection of saline (Figs. 2A and 2B). ADC in the hippocampus was also gradually increased, showing significant changes shortly (10-20 min) after the injection of TGN020 relative to saline control (Fig. 2C). The averaged ADC change in the cortex and the striatum between 30 and 60 min after the injection of TGN020 was significantly increased as compared to injection of saline (Figs. 2D and 2E). Although the averaged ADC change in the hippocampus by TGN020 injection was higher than that by saline injection, no significance was found for this global comparison. Using ex vivo cortical slice with IOS imaging, we found TGN020-influenced tissue swelling induced by intense optogenetic activation of pyramidal neurons (Fig. 3). Our results indicate that DWI is sensitive to the astrocyte volume change by the inhibition of AQP4.Acknowledgements
No acknowledgement found.References
1 Debacker et al, Diffusion MRI reveals in vivo and non-invasively changes in astrocyte function induced by an aquaporin-4 inhibitor, PLoS One, 2020 May 15;15(5):e0229702.
2 Iliff JJ et al, A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β, Sci Transl Med. 2012 Aug 15;4(147):147ra111
3 Chung et al, Determinants of Optogenetic Cortical Spreading Depolarizations. Cereb Cortex . 2019 Mar 1;29(3):1150-1161.
4 Zhou et al, Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb Cortex. 2010 Nov;20(11):2614-24.
Figures