4143
Association of CMR-verified diffuse myocardial fibrosis with depressed myocardial strain in HFpEF
Yi Zhang1 and Xiance Zhao2
1Shanghai General Hospital, Shang Hai, China, 2Philips Healthcare,, Shang Hai, China
1Shanghai General Hospital, Shang Hai, China, 2Philips Healthcare,, Shang Hai, China
Synopsis
Keywords: Myocardium, Quantitative Imaging
HFpEF was seen approximately in half of all hospitalized patients for heart failure and is associated with a poor prognosis. Using CMR, the present study shows that the increased diffuse myocardial fibrosis was associated with the degree of impaired myocardial strain in patients with HFpEF. As diffuse fibrosis is recognized as prognostic markers and reversible. Our findings demonstrate the utility of myocardial strain in assessing patients with HFpEF. The measurement of myocardial strain could be used to inform clinicians about whether HF medications could improve the degree of diffuse myocardial fibrosis in HFpEF.Objectives
The purpose of this study was to investigate the extent of the left ventricular (LV) diffuse myocardial fibrosis and the association with the degree of impaired myocardial strain in patients with HFpEF.Background
The increased diffuse myocardial fibrosis impairs the LV systolic and diastolic function. Previous studies found that the global longitudinal strain (GLS) impacted survival in patients with heart failure with preserved ejection fraction (HFpEF). However, limited data are available regarding the association between the degree of diffuse myocardial fibrosis and the severity of impaired myocardial strain in HFpEF.Methods
Sixty-six consecutive participants with heart failure (HF), and 15 healthy controls underwent cardiac magnetic resonance (CMR) examination (Ingenia 3.0T, Philips Healthcare, Best, the Netherlands). The extracellular volume fractions (ECV) and myocardial strains were compared among the 3 groups. Associations between these two factors were also explored.Results
The patients with HFpEF showed increased myocardial ECV fractions (32.9%±3.7% vs. 29.2%±2.9%, p<0.001) compared with the control group. The patients with HFm+rEF also had increased myocardial ECV fractions (36.8%±5.4% vs. 32.9%±3.7%, p<0.001) compared with HFpEF. The myocardial ECV was significantly correlated with the GLS (r=0.422, p=0.020), global circumferential strain (GCS) (r=0.491, p=0.006), and global radial strain (GRS) (r=-0.533, p=0.002) in the HFpEF groups, but no significant correlation was found in the HFm+rEF group (GLS: r=-0.002, p=0.990; GCS: r=0.153, p=0.372; GRS: r=0.070, p=0.685) .Conclusions
In HF patients, only patients with HFpEF exhibited a significant correlation between increased diffuse myocardial fibrosis and impaired myocardial strain. Diffuse myocardial fibrosis plays a unique role in affecting myocardial strain in patients with HFpEF.Acknowledgements
No acknowledgement found.References
[1]. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The T ask Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 37(27) (2016):2129–2200. [2] Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 296(2006):2209–16. [3] Little WC, Zile MR. HFpEF: cardiovascular abnormalities not just comorbidities. Circ Heart Fail. 5(2012):669–71. [4] Owan TE, Hodge DO, Herges RM, Jacobsen SJ,Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 355(2006):251–9. [5] Pokharel Y, Khariton Y, T ang Y, et al. Association of serial Kansas City Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol.2(12) ( 2017):1315–1321. [6] Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging.13(1 Pt 2) ( 2020):245–257. [7] Nagueh SF. Left ventricular diastolic function: understanding pathophysiology, diagnosis, and prognosis with echocardiography. JACC Cardiovasc Imaging.13 (1 Pt 2)( 2020):228–244. [8] Kanagala P, Cheng A, Singh A, et al. Relationship Between Focal and Diffuse Fibrosis Assessed by CMR and Clinical Outcomes in Heart Failure With Preserved Ejection Fraction[J]. JACC. Cardiovascular imaging.12(11)( 2019): 2291-2301. [9] Mascherbauer J, Marzluf BA, T ufaro C, et al. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging.6(6)( 2013):1056–1065. [10] Kammerlander AA, Donà C, Nitsche C, et al. Feature tracking of global longitudinal strain by using cardiovascular MRI improves risk stratification in heart failure with preserved ejection fraction. Radiology.296(2)( 2020):290–298. [11] Aschauer S, Kammerlander AA, Zotter-T ufaro C, et al. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur J Heart Fail.18(1) ( 2016):71–80. [12] Mascherbauer J, Zotter-T ufaro C, Duca F, et al. Wedge Pressure Rather Than Left Ventricular End-Diastolic Pressure Predicts Outcome in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail.5(11) ( 2017): 795–801. [13] Potter E, Marwick TH. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc Imaging.11(2 Pt 1) ( 2018): 260–274. [14] Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular Magnetic Resonance Myocardial Feature T racking: Concepts and Clinical Applications. Circ Cardiovasc Imaging.9(4) ( 2016): e004077. [15] Cerqueira, M. D. et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 105(2002), 539–542. [16] Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson .14(1) (2012):63-74. [17] Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of Diffuse Myocardial Fibrosis in Heart Failure With Cardiac Magnetic Resonance Contrast-Enhanced T1 Mapping[J]. J Am Coll Cardiol.52(19) ( 2008):1574-80. [18] Borbely A, van der Velden J, Papp Z, et al.Cardiomyocyte stiffness in diastolic heart failure. Circulation.111(2005):774–81. [19] Haaf P, Garg P, Messroghli DR, et al. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson .18(1) ( 2016):89-100. doi: 10.1186/s12968-016-0308-4. [20] Bull S, White SK, Piechnik SK, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 99(2013):932–7. [21] Mewton N, Liu CY, Croisille P, Bluemke D,Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol.57 (2011):891–903. [22] Su M, Lin L Y, Tseng Y, et al. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging.7(10) ( 2014):991-7 [23] Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int.14(2001):299 –30 [24] Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, Wansapura J, Klimeczek P , Al-Khalidi HR, Chung ES, Benson DW, Mazur W. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 3(2010):144–151. doi: 10.1016/j.jcmg.2009.11.006. [25] Kerwin WS, Prince JL. Cardiac material markers from tagged MR images. Med Image Anal.2(1998): 339–353 [26] Tadic M, Pieske-Kraigher E, Cuspidi C, et al. Left ventricular strain and twisting in heart failure with preserved ejection fraction: an updated review[J]. Heart Failure Reviews.,22(3) ( 2017):371-379. [27] Martinez DA, Guhl DJ, Stanley WC, Vailas AC. Extracellular matrixmaturation in the left ventricle of normal and diabetic swine.Diabetes Res Clin Pract .59(2003):1– 9.Figures
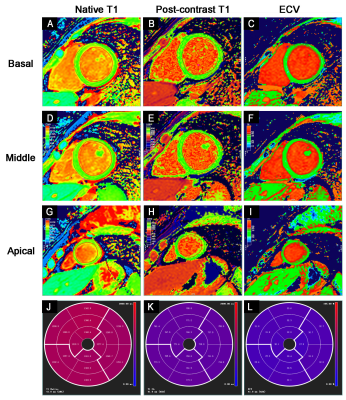
The representative T1 maps from a
healthy control at the left ventricular basal (upper line), middle (second
line), and apical (third line) short-axis segment with a modified look-locker
inversion recovery (MOLLI) sequence showing the native T1 mapping (left row),
post-contrast T1 mapping of the same slice (middle row) and calculated
extracellular volume (ECV) mapping of the same segment (right row). The native
T1 (J), post-contrast T1 (K), and ECV (L) values in a 16-segment model are
displayed.
DOI: https://doi.org/10.58530/2023/4143