4136
MRI relaxometry mapping of hearts extracted post-mortem from acute COVID-19 patients
El-Sayed H. Ibrahim1, Andrii Puzyrenko1, and Ivor Benjamin1
1Medical College of Wisconsin, Milwaukee, WI, United States
1Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Keywords: Myocardium, COVID-19
Cardiac MRI is capable of myocardial tissue characterization using parametric mapping techniques such as T1 and T2 mappings, which are sensitive for detecting diffuse inflammation, fibrosis and edema. These techniques seem adequate for the detection of myocardial changes in COVID-19. In this study, we demonstrated the use of ex vivo MRI mapping techniques for noninvasive myocardial tissue characterization in COVID-19 patients and compared the MRI results to findings from autopsy reports. The proposed approach would allow for better understanding of COVID-19 pathophysiological effects on the myocardium through longitudinal studies that could be conducted without the need for invasive endomyocardial biopsy.Introduction
Cardiac involvement in COVID-19 patients showed to be associated with unfavorable prognosis, which could lead to fatal outcomes as in myocardial injury-induced arrhythmias and sudden cardiac death.1,2 The pathophysiology in COVID-19 is more similar to findings in other chronic diffuse inflammatory syndromes that occur post viral infections, e.g., in HIV.3,4 Although endomyocardial biopsy is considered the gold standard for assessing myocardial involvement, it has limitations of sampling error and limited sensitivity, and it carries inherent risks including rare mortality. Cardiac MRI is capable of myocardial tissue characterization using parametric mapping techniques such as T1 and T2 mappings, which are sensitive markers for detecting diffuse inflammation, fibrosis and edema.5 These techniques seem adequate for the detection of diffuse myocardial changes, as irreversible focused fibrotic or necrotic changes indicated by late gadolinium enhancement may not be present in COVID-19 patients. In a recent study,6 myocardial T1 and T2 measurements showed higher discriminatory values for COVID-19 related involvement than did conventional function and volume parameters. Therefore, MRI mapping techniques may provide a sensitive tool for noninvasive detection of the subset of patients who are at high risk for cardiac complications and myocardial damage. In this study, we investigated the value of ex vivo multiparametric MRI mapping for identifying changes in myocardial tissue composition in COVID-19 autopsied patients and compared the results to autopsy findings.Methods
Nine hearts extracted from consecutive autopsied patients (age = 59±13 y.o., 6 males / 3 females) who died due to acute COVID-19 complications were included in this IRB-approved study. The duration between death and ex vivo MRI exam was 229±15 days, during which the patients’ hearts were preserved in formalin. Myocardial specimens (3-12 samples/heart; 1-2 inches in each dimension) were extracted from different regions of the hearts (Figure 1) and scanned on a 3T GE Premier MRI scanner using Air coil. The imaging protocol included scouting, T1 mapping, and T2 mapping sequences. Optimized imaging parameters for the T1 mapping sequence were as follows: 5(3)3 MOLLI sequence, FIESTA acquisition, TR = 2.8 ms, TE = 1.2 ms, flip angle = 35°, slice thickness = 8 mm, matrix = 160×148, FOV = 350×350 mm2, # averages = 1, and readout bandwidth = 651 Hz/pixel. Optimized parameters for T2 mapping were as follows: fast spin-echo (FSE) sequence, TR = 800 ms, TE = 10-100 ms (4 echoes with 30 ms increments), echo train length = 4, flip angle = 90°, slice thickness = 8 mm, matrix = 224×224, FOV = 350×350 mm2, # averages = 1, readout bandwidth = 326 Hz/pixel. The MRI images were processed using the Circle cvi42 software to generate T1 and T2 maps. Circular regions of interest (ROIs) were drawn at the center of each tissue specimen and average value from different measurements was calculated for each subject. Medical records of the patients were accessed to collect information about lab results of cardiac biomarkers, cardiovascular risk factors, and history of cardiovascular diseases. The autopsy reports were accessed to retrieve information about cause of death and histological analysis findings, including the existence of fibrosis or inflammation. Data are represented as mean±SD. Statistical analysis was conducted on the results, where P<0.05 was considered statistically significant.Results
The main cause of death in the subjects was acute bronchopneumonia that led to respiratory failure. Table 1 summarizes the study population characteristics, MRI parameters, lab results, and autopsy findings and Figure 2 shows MRI anatomical images, T1 maps, and T2 maps. Seven of the subjects had cardiovascular diseases and six of them had cardiac risk factors. The autopsy reports revealed the existence of myocardial fibrosis in 6 subjects and that 1 subject had necrotic cardiac myofibers and acute inflammation. Average T1 and T2 values in these 7 subjects were higher than those in the rest of the subjects: 328±95 ms and 52±5 ms vs 271±34 ms and 50±1 ms, respectively. High-sensitivity cardiac troponin (hsTn) and c-reactive protein (CRP) serum measurements collected shortly before death showed large variability among the studied subjects. The subject with acute inflammation had the highest T1 value of 533 ms. There were no significant differences in T1 or T2 measurements between males and females. It should be noted that the measured myocardium T1 values were significantly less than the range of values typically seen in in vivo scans due to the formalin’s effect on myocardium, as previously reported.7 However, as the hearts from all subjects were preserved in formalin for almost the same duration, relative differences in T1 and T2 values could be adequately used to assess changes in tissue composition, reflecting increased diffuse fibrosis, inflammation and edema.Conclusions
In this study, we demonstrated the use of ex vivo MRI mapping techniques for noninvasive myocardial tissue characterization in COVID-19 patients and compared the MRI results to findings from autopsy reports about tissue fibrosis and inflammation. The proposed approach would allow for better understanding of COVID-19 pathophysiological effect on the myocardium through longitudinal studies that could be conducted without the need for invasive endomyocardial biopsy, with an overarching goal of allowing for prompt medical intervention and improved outcomes in COVID-19 patients.Acknowledgements
Study supported by funding from Medical College of Wisconsin Clinical and Translational Science Institute (CTSI).References
1. Y Xie et al. Nat Med. 2022; 28:583-590.
2. Z Tan et al. Front Cardiovasc Med. 2021;8:795750.
3. A Nalbandian et al. Nat Med. 2021;27:601-615.
4. V Puntmann et al. Nat Med. 2022;28:2117-2123.
5. N Galea et al. J Cardiovasc Magn Reson. 2021;23:68.
6. V Puntmann et al. JAMA Cardiol. 2020;5:1265-1273.
7. Ebata et al. BMC Med Imaging. 2021; 21:134.
Figures
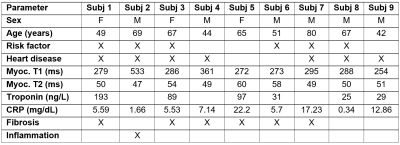
Table 1. Cardiac MRI
parameters, lab results and autopsy findings in studied COVID-19 patients. Abbreviations:
hsTn, high-sensitivity troponin; CRP, C-reactive protein.
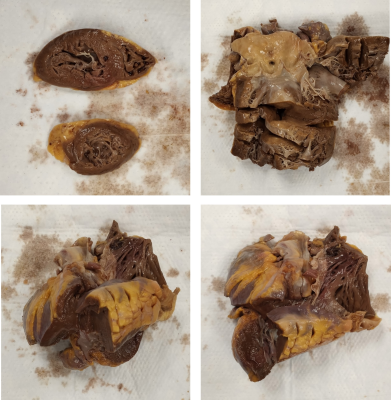
Figure 1. Myocardium tissue specimens from COVID-19
autopsied patients used for ex vivo MRI imaging.
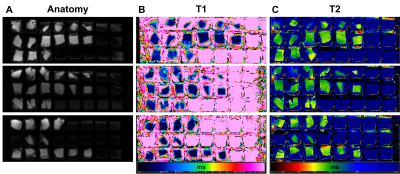
Figure 2. (A) Anatomical, (B) T1-mapping, and (C)
T2-mapping MRI images of myocardial tissue specimens from COVID-19 autopsied
patients. Note changes in mapping values (color) among different specimens.
DOI: https://doi.org/10.58530/2023/4136