4130
Free-breathing, ECG-Free, Myocardial T1 Mapping: Initial Feasibility Experiments of Cardiac MR Multitasking on GE Systems1GE HealthCare, Menlo Park, CA, United States, 2Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Department of Radiology, McGill University Health Centre, Montreal, QC, Canada, 4GE HealthCare, Barcelona, Spain, 5Department of Bioengineering, University of California in Los Angeles, Los Angeles, CA, United States, 6Area 19, Montreal, QC, Canada, 7Departments of Medicine and Radiology, Stanford University, Stanford, CA, United States, 8GE HealthCare, Munich, Germany
Synopsis
Keywords: Myocardium, Quantitative Imaging, T1 Mapping
Quantitative imaging biomarkers such as T1 are promising for assessing focal and diffuse myocardial pathologies across different sites and scanners. However, the lack of reliable T1 mapping methods across different scanner platforms reduces patient access to robust quantitative imaging. CMR Multitasking has shown promise for non-ECG and free-breathing quantitative imaging in the heart, primarily at 3T. This work is an initial feasibility study implementing the CMR Multitasking on GE 1.5T MR scanners. Radial trajectories, self-gating/training data, and the CMR Multitasking framework together provided co-registered T1 and cine maps in a 1-min, free-breathing, non-ECG CMR scan.Introduction
Quantitative imaging biomarkers (e.g., T1) are promising for assessing focal and diffuse myocardial pathologies across different sites and scanners. However, the lack of reliable T1 mapping methods across different scanner platforms reduces patient access to robust quantitative imaging. Meanwhile, conventional T1 mapping and cine protocols are often challenging in elderly and obese patients because they rely on unreliable mechanisms deal with cardiac motion (e.g., ECG triggering and breath-holding). CMR Multitasking1-4 has demonstrated T1 cine mapping without ECG gating and breath-holding, primarily at 3T. We implemented a 2D single-slice free-breathing, non-ECG, T1 cine mapping technique (i.e., 2D T1 CMR Multitasking) on GE 1.5T systems, with a dual flip angle scheme to enable robust and repeatable T1 mapping3-5.Methods
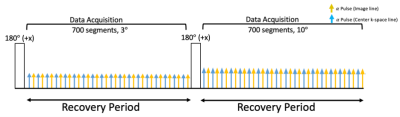
Sequence Design: The proposed continuous 2D T1 CMR Multitasking sequence uses a radial trajectory modified to collect low-rank tensor training data interleaved with image data. A golden angle (111.25°) angular increment was used between consecutive imaging readouts. The training data is acquired every other readout. Non-selective IR pulses are employed to generate the T1 contrasts (i.e., Fig. 1). The continuous acquisition sequence collects FGRE readouts after the preparation pulses. FGRE excitation alternates between 3° and 10° for each inversion recovery period to allow for B1+ robust T1 mapping3-5.Image Reconstruction and T1 Estimation: The images acquired in the 2D T1 CMR Multitasking framework can be represented as a 5-way tensor 𝒜 with voxel location index $$$\textbf{r}=[x,y,z]$$$, a T1 recovery dimension, a cardiac motion dimension, a respiratory motion dimension, and a flip angle dimension. The standard Multitasking reconstruction pipeline1-4 is followed. The signal equation at the kth recovery period of the 2D T1 CMR Multitasking sequence is
$$s(A,B,T_1, \beta) = A\frac{1-e^{-TR/T1}}{1-e^{-TR/T1}cos(\beta\alpha_k)} [1 + (BQ_k-1)(e^{-TR/T1}cos(\beta\alpha_k))^n] \cdot sin(\beta\alpha_k), \quad (1)$$
with amplitude factor $$$A$$$, IR pulse efficiency $$$B$$$, FGRE readout interval $$$TR$$$, flip angle for the $$$k^{th}$$$ recovery period B1+ field weights $$$\beta$$$ (to account for B1+ inhomogeneity), and recovery time point $$$n=1,2,3, ..., N$$$, ( $$$N$$$ is the number of FGRE pulses in a recovery period). The $$$Q_k$$$ absorbs the effects of having inverted the magnetization from the steady-state for the previous recovery period’s excitation flip angle. Assuming a steady-state established at the final readout of each recovery period, $$$Q_k$$$ is expressed as
$$Q_k = \frac{1-e^{-TR/T1}cos(\beta\alpha_{k})}{1-e^{-TR/T1}cos(\beta\alpha_{k-1})}. \quad (2)$$
A two-step fitting approach4 is used to determine the parameter maps. Step 1 estimates from Eq.(1), and step 2 uses the known to fit T1 from the 3° recovery curve only, for which the Look–Lockereffect is reduced.
Data Collection: Four human volunteers (2 males; 2 females) were imaged on a 1.5T MR450w scanner (GE HealthCare, Waukesha, WI) at basal, mid, and apical short-axis (SAX) slices. 2D T1 CMR Multitasking pulse sequence parameters were: TR/TE=3.6 ms/1.8 ms, recovery period = 2.5 s, spatial resolution=1.7x1.7x8.0 mm3, scan time=1min, 20 cardiac bins, 6 respiratory bins. Reference bSSFP cine (1.7x1.7x8.0 mm3) and 2D T1 maps with MOLLI (2.0x2.0x8.0 mm3) were acquired at same SAX slices at end-diastole during end-expiration breath-holds using bSSFP readout and the 5(3s)3 heart-beat pattern.
Results
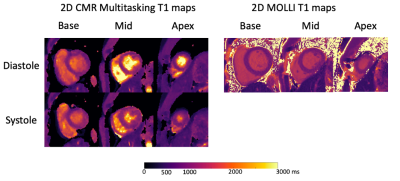
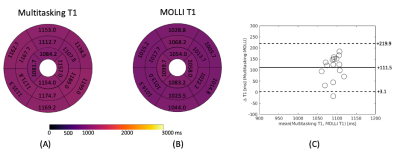
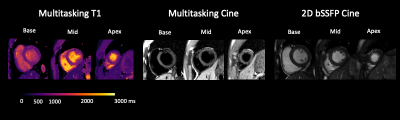
T1 mapping images acquired from the 2D T1 CMR Multitasking sequence are shown in Fig. 2, along with the reference 2D diastolic T1 images acquired from MOLLI. Fig. 3A-B depicts the summary statistics for segment-wise T1 values in four human volunteers in Multitasking and MOLLI. Multitasking T1 maps reported 1147.4$$$\pm$$$93.5ms, which is slightly higher than the MOLLI T1 measurements 1035.9$$$\pm$$$59.9ms. Fig. 3C shows the Bland-Altman plot of the multitasking segment-wise T1 values and reference myocardial T1 values. Fig. 4 also shows the T1 cine maps from the 2D T1 CMR Multitasking.Discussion
Myocardial T1 maps were acquired at basal, mid, and apical SAX slices using a 2D T1 CMR Multitasking sequence on a GE 1.5T system. The myocardial T1 quantification showed positive bias compared to reference MOLLI measurements but is close to the reported T1 values measured by SMARTT1Map on 1.5T GE Systems6,7, and showed a similar trend to previous Multitasking findings at 3T3-4. Cine mapping improves the reliability of quantification by allowing for cardiac phase selection and would also allow for analyzing changes of myocardial T1 across their cardiac cycle.Conclusion
Initial feasibility results show that CMR Multitasking is compatible with GE MR systems. The image quality of T1 estimation from CMR Multitasking is comparable to the existing vendor provided cardiac T1 mapping techniques. The free-breathing and non-ECG feature of Multitasking may make this technique widely adoptable in multiple sites. Future studies will involve larger healthy subject and patient cohorts for across site and across scanner research.Acknowledgements
No acknowledgement found.References
[1] Christodoulou, A. G., Shaw, J. L., Nguyen, C., Yang, Q., Xie, Y., Wang, N., & Li, D. (2018). Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nature biomedical engineering, 2(4), 215-226.
[2] Cao, T., Wang, N., Kwan, A. C., Lee, H. L., Mao, X., Xie, Y., ... & Li, D. (2022). Free‐breathing, non‐ECG, simultaneous myocardial T1, T2, T2*, and fat‐fraction mapping with motion‐resolved cardiovascular MR multitasking. Magnetic Resonance in Medicine, 88(4), 1748-1763.
[3] Serry, F. M., Ma, S., Mao, X., Han, F., Xie, Y., Han, H., ... & Christodoulou, A. G. (2021). Dual flip‐angle IR‐FLASH with spin history mapping for B1+ corrected T1 mapping: Application to T1 cardiovascular magnetic resonance multitasking. Magnetic Resonance in Medicine, 86(6), 3182-3191.
[4] Mao, X., Lee, H. L., Hu, Z., Cao, T., Han, F., Ma, S., ... & Christodoulou, A. G. (2022). Simultaneous Multi-slice Cardiac MR Multitasking for Motion-Resolved, Non-ECG, Free-Breathing T1-T2 Mapping. Frontiers in Cardiovascular Medicine, 267.
[5] Zhou, R., Weller, D. S., Yang, Y., Wang, J., Jeelani, H., Mugler III, J. P., & Salerno, M. (2021). Dual‐excitation flip‐angle simultaneous cine and T1 mapping using spiral acquisition with respiratory and cardiac self‐gating. Magnetic Resonance in Medicine, 86(1), 82-96.
[6] Burkhardt, B. E. U., Menghini, C., Rücker, B., Kellenberger, C. J., & Valsangiacomo Buechel, E. R. (2020). Normal myocardial native T1 values in children using single‐point saturation recovery and modified look–locker inversion recovery (MOLLI). Journal of Magnetic Resonance Imaging, 51(3), 897-903.
[7] Matsumoto, S., Okuda, S., Yamada, Y., Suzuki, T., Tanimoto, A., Nozaki, A., & Jinzaki, M. (2019). Myocardial T1 values in healthy volunteers measured with saturation method using adaptive recovery times for T1 mapping (SMART1Map) at 1.5 T and 3 T. Heart and vessels, 34(11), 1889-1894.
Figures