4125
Amide proton transfer-weighted imaging histogram analysis to predict pathological extramural venous invasion in rectal adenocarcinoma1The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China, 2Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Digestive, Cancer, Rectal neoplasms
This study evaluate amide proton transfer-weighted (APTw) derived whole-tumour histogram analysis parameters in predicting pathological extramural venous invasion (pEMVI) positive status of rectal adenocarcinoma (RA). The method is to calculate APTw histogram parameters between the pEMVI-negative and positive groups, assess the independent risk factors and diagnosis performance. Our results demonstrated that RA with pEMVI-positive status is associated with higher APTw-SI. The best prediction for EMVI involvement was obtained with a combined model of histopathological factors and APTw histogram parameters. Therefore, the APTw histogram analysis may represent a valuable non-invasive tool in predicting pEMVI positive status of RA.Introduction
Colorectal cancer is the third most common cancer, with rectal cancer (RC) accounting for 30–35% of these cases [1]. Extramural venous invasion (EMVI) refers to tumor cells invading into blood vessels located beyond the muscularis propria and is an independent prognostic factor of worse oncologic outcomes in patients with RC [2-4]. Besides, the choice of therapeutic strategies is associated with the preoperative assessment of EMVI status [5-7]. Therefore, a non-invasive and accurate EMVI diagnosis is critical for estimating prognosis and deciding an optimal treatment strategy for patients with RC. APTw imaging is a chemical exchange saturation transfer process, and its signal intensity (SI) originates mainly from the exchange of protons between bulk water and endogenous mobile proteins and peptides. Recent studies concerning RC have showed that APTw imaging helps to predict histopathological grade, treatment response to neoadjuvant chemoradiotherapy, and assess EMVI status [8-10]. Nonetheless, several studies have reported conflicting results in evaluating pEMVI status [9]. Histogram is a first-order statistical method that measures the properties of the individual pixel value [11]. Therefore, the purpose of this study was to evaluate APTw derived whole-tumour histogram analysis parameters in predicting pEMVI positive status of RC.Methods
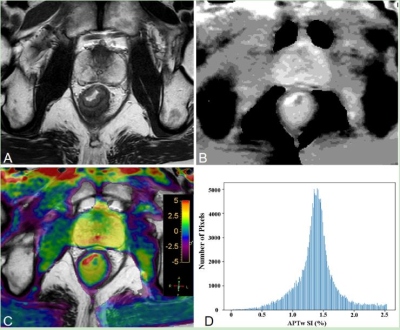
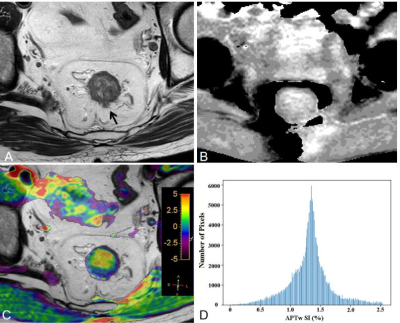
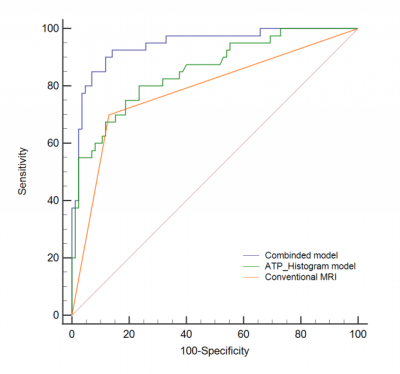
Pre-operative MR including APTw imaging of 125 patients with RA (mean 61.4±11.6 years) were retrospectively analysed. Two radiologists reviewed each case’s EMVI status based on the MR-based modified five-point scale system with conventional MR images. They were blinded to the clinical and pathological information. The APTw histogram parameters of primary tumors were obtained automatically using whole-tumour volume histogram analysis, including APTw-minimum, APTw-maximum, APTw-mean, APTw-10%, APTw-90%, interquartile range, APTw-median, range, mean absolute deviation (MAD), robust mean absolute deviation (RMAD), root mean squared (RMS), energy, total energy, entropy, kurtosis, skewness, uniformity, and variance. Figure 1 and 2 show representative pEMVI-negative and pEMVI-negative RA cases, respectively. The independent risk factors markedly correlated with pEMVI-positive status were assessed using univariate and multivariate logistic regression analyses. To evaluate the probability of EMVI involvement, two multivariable logistic model was built; one model only included APTw histogram variables associated with EMVI involvement, the other model included both histopathological and APTw histogram variables. Receiver operating characteristic (ROC) curve analysis was used to evaluate the diagnosis performance of conventional MRI and two multivariate model for predicting pEMVI status (Fig 3). The AUCs were compared using the Delong method.Results
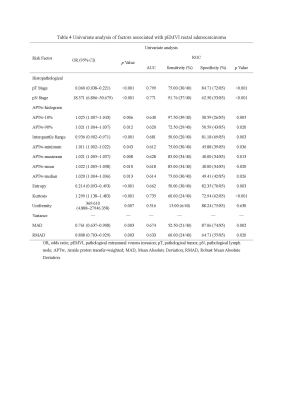
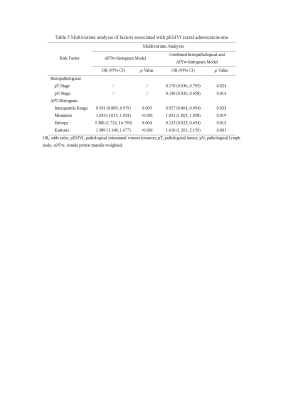
Univariate analysis demonstrated that an advanced tumor stage, lymph node involvement, APTw-10%, APTw-90%, interquartile range, APTw-minimum, APTw-maximum, APTw-mean, APTw-median, entropy, kurtosis, mean absolute deviation (MAD), and robust MAD were significantly related to pEMVI-positive status (all p<0.05) (Table 1). Multivariate analysis demonstrated that an advanced tumor stage (OR=0.170, p=0.024), lymph node involvement (OR=0.130, p=0.014), interquartile range (OR=0.927, p=0.033), APT-minimum (OR=1.031, p=0.019), entropy (OR=0.125, p=0.012), and kurtosis (OR=1.610, p=0.001) were the independent risk factors enabling prediction of pEMVI-positive status (Table 2). The AUCs for diagnostic ability of conventional MRI assessment, the APTw-histogram model, and the combined model (including histopathological and APTw-histogram variables) were 0.785, 0.853, and 0.944, respectively. The combined model outperformed the APTw-histogram model (p=0.0041) and the conventional MRI assessment (p=0.0005).Discussion
Our results demonstrated that RA with pEMVI-positive status is usually associated with relatively higher APTw-SI, an increased concentration of mobile macromolecules (protein and peptides) caused by tumor proliferation and increased blood in tumor vessels could be potential sources of high APTw-SI [12]. The finding is inconsistent with Li et al.’s result. Inconsistent ROI selection methods and patient sample selection may help to explain the discrepant findings. In Li et al.'s study, the ROIs were placed on three slices for analysis [9]. Tumors are biologically heterogeneous. The mean value obtained from the ROI-based approach will likely underestimate tumor heterogeneity. Besides, the ROI approach might impact the repeatability of tumor segmentation. In addition, Li et al.’s study contained a relatively small study population (n=95) and an unbalanced population distribution, with only 25 EMVI-positive cases [9]. The best prediction for EMVI involvement was obtainted with a combined model of histopathological (advanced tumor stage and lymph node metastasis) and APTw histogram variables (area under the curve, 0.944). In RA, pEMVI-positive status correlates with increased tumor aggressiveness properties such as local invasion and regional lymph node involvement [13,14]. These findings could help explain that the advanced tumor stages and pN1-2 stage are independent factors that enable the prediction of pEMVI-positive RA. Kurtosis represents the peakedness of the distribution and measures the probability distribution's shape, while entropy measures the irregularity of gray-level distribution. Kurtosis with markedly higher values in pEMVI-positive RA was the best independent predictor for pEMVI status (OR=1.61, p=0.001) in combined model. The increased kurtosis value of APTw histograms reflects tumor heterogeneity regarding the difference in mobile proteins and peptides in the proliferating tumor cells, which might be interpreted by increased cellular density and cytoplasmic protein and angiogenesis in pEMVI-positive status RA, as discussed earlier. APTw-minimum and interquartile range describe the lowest and the central 50% of APTw values, respectively. It should be noted that APTw-minimum is more likely influenced by random noise, which might reduce stability [15].Conclusion
Whole-tumour histogram analysis of APTw images combined histopathological factors showed better diagnosis efficiency in predicting EMVI involvement in RA.Acknowledgements
No acknowledgement found.References
1. Siegel RL, Miller KD, Fuchs HE et al. Cancer statistics, 2022. CA Cancer J Clin. 2022; 72 (1): 7-33. 2. Yeo DM, Oh SN, Lee MA, et al. The development and validation of a predictive model for recurrence in rectal cancer based on radiological and clinicopathological data. Eur Radiol. 2021; 31 (11): 8586-8596.
3. Zhao Q, Wan L, Zou S, et al. Prognostic risk factors and survival models for T3 locally advanced rectal cancer: what can we learn from the baseline MRI? Eur Radiol. 2021; 31 (7): 4739-4750.
4. Zhang XY, Wang S, Li XT, et al. MRI of Extramural Venous Invasion in Locally Advanced Rectal Cancer: Relationship to Tumor Recurrence and Overall Survival. Radiology. 2018; 289(3): 677-685.
5. Wo JY, Anker CJ, Ashman JB, et al. Radiation Therapy for Rectal Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2021; 11(1): 13-25.
6. Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28(suppl_4): iv22-iv40.
7. Kennedy ED, Simunovic M, Jhaveri K, et al. Safety and Feasibility of Using Magnetic Resonance Imaging Criteria to Identify Patients With "Good Prognosis" Rectal Cancer Eligible for Primary Surgery. JAMA Oncol. 2019; 5(7): 961-966.
8. Nishie A, Takayama Y, Asayama Y, et al. Amide proton transfer imaging can predict tumor grade in rectal cancer. Magn Reson Imaging. 2018; 51:96-103.
9. Li J, Lin L, Gao X, et al. Amide Proton Transfer Weighted and Intravoxel Incoherent Motion Imaging in Evaluation of Prognostic Factors for Rectal Adenocarcinoma. Front Oncol 2022 Jan 3;11:783544.
10. Nishie A, Asayama Y, Ishigami K, et al. Amide proton transfer imaging to predict tumor response to neoadjuvant chemotherapy in locally advanced rectal cancer. Gastroenterol Hepatol. 2019; 34 (1): 140-146.
11. Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014; 111 (12): 2205-13.
12. Zheng S, van der Bom IMJ, Zu Z, et al. Chemical exchange saturation transfer effect in blood. 2014; 71 (3):1082-92.
13. Yu X, Song W, Guo D, et al. Preoperative Prediction of Extramural Venous Invasion in Rectal Cancer: Comparison of the Diagnostic Efficacy of Radiomics Models and Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Front Oncol. 2020 Apr 9; 10: 459.
14. Chen Y, Yang X, Wen Z, et al. Association between high-resolution MRI-detected extramural vascular invasion and tumour microcirculation estimated by dynamic contrast-enhanced MRI in rectal cancer: preliminary results. BMC Cancer. 2019; 19 (1): 498.
15. Cyril Höschl Iv JF. Robust histogram-based image retrieval. Pattern Recogn Lett 2016; 69: 72-81.
Figures