4123
Longitudinally Evaluation of Locally Advanced Rectal Carcinoma Treated with Preoperative Chemotherapy using Time-Dependent Diffusion MRI1Department of Radiology, West China Hospital, Sichuan University, Cheng du, China, 2Colorectal Cancer Center,Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China, 3Philips Healthcare, China, Beijing, China, 4Department of Radiology, West China Hospital, Sichuan University, Chengdu, China
Synopsis
Keywords: Cancer, Microstructure, rectal cancer
The effectiveness of neoadjuvant Chemotherapy (NACT) is most commonly assessed by the change in tumor size based on conventional rectal cancer imaging. However, morphologic changes often occur later during treatment. Time-Dependent Diffusion MRI (TD MRI) is a novel MRI tool for depicting cellular microstructures. This study aimed to investigate the feasibility of Time-Dependent Diffusion MRI–based microstructural mapping for noninvasively characterizing cellular properties of LARC during NACT.Synopsis
The effectiveness of neoadjuvant Chemotherapy (NACT) is most commonly assessed by the change in tumor size based on conventional rectal cancer imaging. However, morphologic changes often occur later during treatment. Time-Dependent Diffusion MRI (TD MRI) is a novel MRI tool for depicting cellular microstructures. This study aimed to investigate the feasibility of Time-Dependent Diffusion MRI–based microstructural mapping for noninvasively characterizing cellular properties of LARC during NACT.Summary of Main Findings
In this study, we performed a longitudinal evaluation of locally advanced rectal carcinoma treated with NACT using Time-Dependent Diffusion MRI (TD MRI). TD MRI–based microstructural mapping is well suitable for depicting cellular microstructure changes during NACT.Introduction
Locally advanced rectal carcinoma (LARC) ranks as the third most frequent malignancy and the second most common cause of cancer-related death globally [1]. Pathologic complete response (pCR) of LARC to neoadjuvant Chemotherapy (NACT) is strongly correlated with improved disease-free survival and overall survival. Because of a small proportion of patients with LARC could achieve pCR [2], early assessment of the treatment response is essential. The participant with predicted non-pCR may be directed toward more aggressive or novel therapies at an early stage. Therefore, noninvasive methods that can assess the response to LARC in the early stage of the treatment are highly desired.Time-Dependent Diffusion MRI (TD MRI) has demonstrated unique advantages in depicting cellular microstructure such as cell size and density using an oscillating gradient spin echo (OGSE) diffusion method [3, 4]. In this study, the response to NACT of LARC was monitored longitudinally using TD MRI–based microstructural mapping and standard measurements of apparent dispersion coefficient (ADC).
Image Acquisition
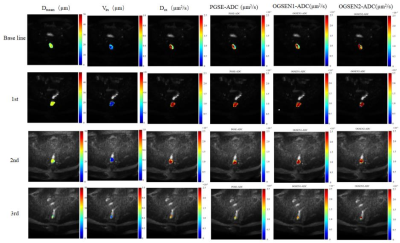
TD MRI technique requires acquisition of diffusion MRI signals at varying diffusion times by using a combination of oscillating gradient spin-echo (OGSE) and pulsed gradient spin-echo sequences to measure diffusion within solid tumors. All scans were performed with a 3.0-T MRI scanner (Ingenia Elition, Philips Healthcare, the Netherlands) with maximum gradient of 45 mT/m and maximum slew rate of 220 mT/m/msec, with the participant in the supine position and using an external phased-array body coil. An in-house OGSE diffusion MRI sequence was implemented with trapezoid-cosine gradients and echo-planar imaging acquisition. OGSE data were acquired at OGSE N2 (33 Hz, duration of diffusion gradient = 60.9 msec, two cycles, b = 0, 60, 120, 180 sec/mm2) and OGSE N1 (17 Hz, duration of diffusion gradient = 60.9 msec, one cycle, b = 0, 250, 500, 750 sec/mm2), and pulsed gradient spin-echo at diffusion duration and separation of 15.9 and 115.1 msec, respectively (b = 0, 300, 600, 900, 1200 sec/mm2). The following parameters were used for both sequences: three diffusion directions; repetition time msec/echo time msec, 5000/140; field of view, 220 × 220 mm; in-plane resolution, 2.75 × 2.75 mm; number of slices, 6; and section thickness, 5 mm. MR images were collected at 4-time points, including based line (before treatment), 1st (after 7 days), 2nd (after 1 cycle), and 3rd (after 2 cycles).Results
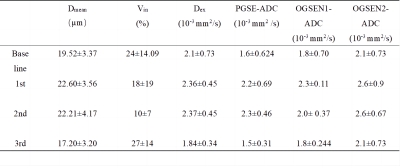
The effectiveness of NACT is most commonly assessed by the change of tumor size based on conventional rectal T2 weighted MRI. However, morphologic changes often occur later during treatment. TD MRI not only provide conventional ADC value, but also cellular microstructural information, such as cell diameter (Dmean), intracellular volume fraction (Vin), and extracellular diffusivity (Dex) through Imaging Microstructural Parameters Using Limited Spectrally Edited Diffusion (IMPULSED) approach (Figure 1). Although tumor size remained unchanged at the early stage, cellular microstructures make the response to the treatment (Figure 2).Discussion
One of the potential applications for MR cellular microstructure imaging is that cellular microstructure could provide earlier and more specific assessments of tumor therapeutic response to various treatments. Because changes at the cellular level (e.g., cell size and intracellular volume fraction) are usually early response of cancer cells to treatment, characterization of cell size and intracellular diffusivity may provide a unique means to assess tumor status. Therefore, TD MRI shows the potential to assess the effectiveness of NACT before morphologic changes.Conclusion
Time-Dependent Diffusion MRI is a valuable tool in the non-invasive longitudinally evaluation of locally advanced rectal carcinoma treated with neoadjuvant chemotherapy.Acknowledgements
No acknowledgement found.References
[1] R.L. Siegel, K.D. Miller, H.E. Fuchs, A. Jemal, Cancer Statistics, 2021, CA Cancer J Clin 71(1) (2021) 7-33. https://doi.org/10. 3322/caac.21654
[2] M. Maas, P.J. Nelemans, V. Valentini, et al., Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data, Lancet Oncol. 11 (2010) 835–844, https://doi.org/10.1016/s1470-2045(10)70172-8.
[3] Wu, D., Jiang, K., Li, H., Zhang, Z., Ba, R., Zhang, Y., Hsu, Y. C., Sun, Y., & Zhang, Y. D. (2022). Time-Dependent Diffusion MRI for Quantitative Microstructural Mapping of Prostate ancer. Radiology, 303(3), 578–587. https://doi.org/10.1148/radiol.211180.
[4] Jiang X., Li H., Sean P. Devan., John C. Gore, Xu J. (2021). MR cell size imaging with temporal diffusion spectroscopy. Magnetic Resonance Imaging 77, 109–123. https://doi.org/10.1016/j.mri.2020.12.010
Figures